18 Cost-Effective Staging of Breast Cancer
Overview
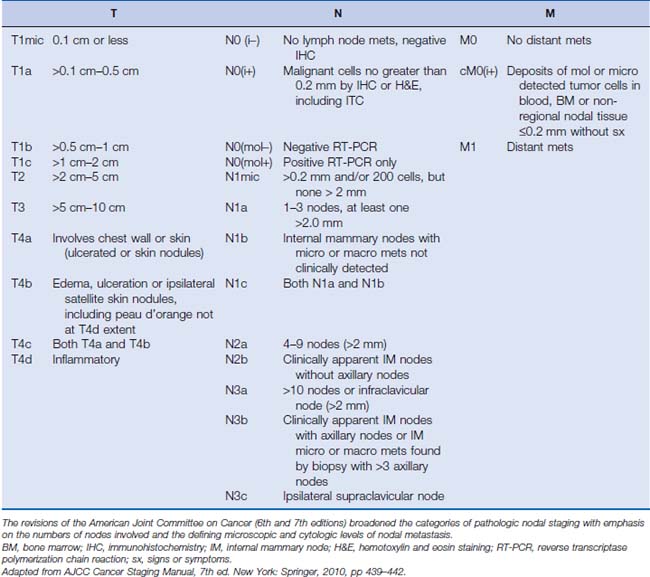
At diagnosis, significant effort is invested to accurately determine the stage of the breast cancer to both inform prognosis and guide therapeutic interventions. Breast cancer staging follows the traditional TNM classification. Recent revisions to the classification schema have delineated greater detail of tumor presentations and extent of pathologic nodal involvement (Table 18-1).
The technologic advances that have made all the aforementioned improvements possible come with an increased expense to the overall breast cancer cost burden. Increasingly, countries outside the United States are requiring cost-effectiveness data at the time of submission for funding/reimbursement of a new diagnostic modality within its socialized or third-party payer system. However, in the United States, new modalities for staging are frequently available and potentially reimbursed before data availability or in spite of negative data for cost-effectiveness. In fact, cost-effectiveness is specifically not used by the Health Care Financing Administration (HCFA) for making decisions about what new technologies will be eligible for Medicare reimbursement.1 Thus, the level of rational decision making for appropriate and (hopefully) cost-effective staging often remains with the health care providers and the patient.
A cost-effective analysis calculates the cost-per-unit benefit of an intervention. It provides a numerical result without passing judgment as to the value of that result or whether we should interpret the result as being worthy. In contrast, a cost-benefit analysis quantifies cost and benefit in the same units, and it queries which is greater—the cost or the benefit. Efficacy of an intervention is the value that an intervention offers when performed under ideal conditions. Conversely, effectiveness is the value of the intervention under routine conditions. Because of the very nature of advanced technologic staging techniques, these issues are of paramount importance for maintaining clarity for data interpretation and decision making.2
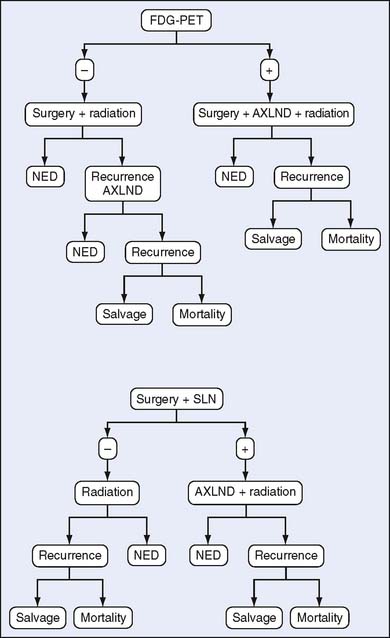
Cost-effectiveness can be determined in several ways. Prospective and retrospective observational studies of changes in clinical management and their outcomes constitute a common method. However, these studies are expensive, require lengthy follow-up, and can be so large that they are impractical to conduct. Alternatively, more sophisticated theoretical modeling can be used. One form of modeling focuses on groups with similar characteristics and uses the “decision tree analysis” or Markov modeling to compare interventions. This useful methodology is limited by requiring knowledge or making assumptions for procedural costs, sensitivity, and specificity of the techniques being compared, as well as the prevalence of outcome being investigated (i.e., nodal metastasis) to drive the model. Outcomes of costs versus survival, disease-free survival, or other desired endpoints are generated, thus enabling comparison among the modalities (Fig. 18-1). Alternatively, microsimulation models can be used to represent the history of disease in an individual and assess cost-effectiveness of one intervention over another, allowing for adaptation of the model to the results of the intervention on the individual. Micro-models are considered more robust and more capable of imitating the data obtained from observational studies3 (Table 18-2).
Table 18-2 Comparison of Results of Macro- and Micro-Modeling for Cost-Effectiveness in Breast Cancer
| Cited Reference | Modeling Method | Cost-Effectiveness |
|---|---|---|
| Rosenquist and Lindfors4 (1998) | Decision tree within Markov model | $18,800–$16,100 |
| Salzmann et al5 (1997) | Markov model with Monte Carlo sensitivity analysis | $105,000 |
The input of different assumptions into a mathematical model and the robustness of the model used can greatly impact the results. Two modeling studies of mammograms for women aged 40–49 registered very different results.
Local Staging of Newly Diagnosed Breast Cancer
In-Breast Staging
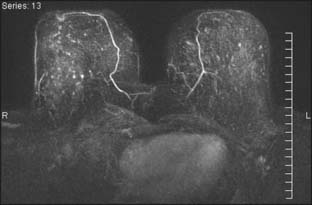
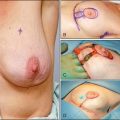
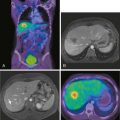
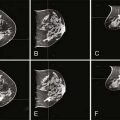
Several reports published in the 1990s demonstrated the ability of breast MRI to improve the accuracy of radiologic imaging to determine the extent of cancer in the affected breast.6–10 Whereas mammography and ultrasound may underestimate tumor size, particularly in very dense breasts, MRI is associated with more accurate correlation with final pathologic size.9,11,12 Histologic differences within breast cancer may impact the added usefulness of MRI. Invasive lobular carcinoma (ILC) is notorious for being occult with standard imaging. MRI is reported to enhance detection of ILC from 34% with mammogram alone to 96% with MRI and to change surgical management 50% of the time.10,13 However, these same studies noted that ultrasound improved detection up to 86% and MRI did not add to detection in a statistically significant manner (Fig. 18-2). A more recent study has correlated histologic grade with MRI-enhanced tumor staging in the breast. High-grade tumors correlated with pathologic tumor size; however, low-grade tumors had significantly poorer correlation with pathologic size and were overestimated in their extent in 13% of cases.12
In addition to evaluation of tumor size, MRI of the affected breast can improve preoperative detection of mammographic and clinically occult tumor foci distinct from the index lesion. Multifocality and multicentricity have been reported in 16% to 37% of cases in which preoperative MRI was added to conventional radiologic studies, with the MRI result changing surgical management in 11% to 14%.8,14,15 Moreover, MRI is a very useful tool for identifying occult primary lesions in the setting of axillary nodal metastasis, potentially permitting breast conservation in lieu of the prior standard of mastectomy and chest wall radiation therapy.16–18
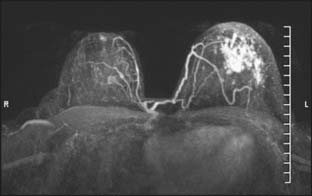
MRI as an in-breast staging tool for newly diagnosed breast cancer is limited by the lack of specificity it offers and the limitation of readily available MR-guided localization and biopsy of detected areas of enhancement. Benign lesions and normal breast tissue may enhance in a manner that is concerning for the presence of additional foci. Second-look ultrasound may identify the lesion and clarify the nature of the enhancement, however, not in all cases. The ability to perform subsequent MR-guided biopsy with technical expertise is limited at present, resulting in a diagnostic dilemma for the patient. MRI specificity ranges from 30% to 99% in the literature, and positive predictive value is reported at 30% to 74%.8,10,19 Overestimation of the extent of disease of the primary lesion has been reported to be 21% when used alone. Interpretation of the MRI in the context of the mammogram and clinical examination were more sensitive than any one test or combination of other tests, and it lowered the overestimation of disease to 6%.10 With these uncertainties about MRI breast staging, it is critical that histologic determination of areas of uncertainty be performed before surgical decision making ensues and that MRI findings alone should not guide recommendation for mastectomy in a clinical scenario otherwise acceptable for breast conservation (Fig. 18-3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree