Biological Effects of Ionizing Radiation
Very high levels of ionizing radiation, such as that from a nuclear explosion, will cause severe cell damage or cell death. Adverse health impacts include early death, within days or a few weeks, as a result of acute exposure whereas longer-term consequences include development of cancer, or genetic maldevelopment as a result of damage to the reproductive cells. It is more difficult to predict the effects of low-level doses of ionizing radiation such as cosmic radiation or medical x-rays because of the individual variability in the body’s self-repair processes. Indeed, several health effects have been suggested at low doses and dose rates, including that the effect of radiation on human health is not linear, but is either a J-shaped curve with exposure being beneficial at low doses (
13,
14); or in contrast is increased due to nontargeted effects where cells not directly traversed by radiation tracks are responsible for malignancy (
15,
16).
Biological effectiveness depends on the spatial distribution of the energy imparted and the density of the ionizations per unit path length of the ionizing particles. The energy loss per unit path length of a charged particle is referred to as the stopping power, whereas the energy deposited is referred to as linear energy transfer (LET).
The ionization process in living tissues consists of atomic and molecular excitations, and ejecting bound electrons from the cellular molecules, leaving behind chemically active radicals that are the source of adverse changes. Many of the radicals resulting from radiation injury are similar to those produced in normal metabolic processes, for which the cell has developed recovery mechanisms needed for long-term survival (
17). The number of ionization events per particle passage is related to the physical processes by which particle kinetic energy is transferred to the cellular bound electrons (
2). The rate at which ions produce electrons in isolated cells is important because repair of a single event is relatively efficient unless many events occur within the repair period (
14).
The substantive target of radiation injury is considered to be the DNA structure that may be changed or injured directly by a passing ionizing particle (
2). DNA damage consists of simple types with a single base damage or break in the DNA sugar-phosphate backbone, termed a
single strand break, to complex types where two or more damages occur in a single helical turn of DNA. The spectrum of DNA damages shifts from simple to more complex as the LET is increased (
18). Double-strand breaks (DSB), defined as one or more breaks on opposing sides of the DNA sugar-phosphate backbone within 20 base pairs of each other, are expected to be the most detrimental form of DNA damage leading to various forms of mutation including gene deletion and chromosomal aberrations. For high-LET radiation, most DSB are highly complex involving base damage and other breaks near a DSB.
The ability of the cell to repair the effects of ionization depends on the class of DNA lesion (simple or complex) and in part on the number of such events occurring within the cell from the passage of a single particle, and the rate at which such passages occur. There are two major pathways of DSB repair in vertebrae (
19): (i) nonhomologous end-joining (NHEJ) and (ii) homologous recombination (
HR). NHEJ is an error-prone form of repair and is dominant in
the prereplication phase of the cell cycle and in resting cells. This process involves removal of damaged regions near the initial break and ligation of the remaining DNA ends.
HR is a high-fidelity form of DNA damage repair, acting during DNA replication and mitosis, and requires a sister chromatid to act as a template for the synthesis of DNA during repair.
In recent years, there has been increased focus on non-DNA targets for harmful biological effects of radiation (
15,
16). These include oxidative damage in the cytoplasm and mitochondria, and aberrant cell signaling processes that disrupt normal cellular processes such as the control of cellular growth factors, the tissue microenvironment, and DNA replication. These so-called nontargeted effects can be both mutagenic and carcinogenic.
Chromosome Aberrations
Tissue cells may be damaged by physical agents such as heat, cold, vibration, and radiation. Throughout life, there is a continuous ongoing cycle of cell damage and repair utilizing the body’s self-repair mechanisms. During the repair process, gene translocation and other chromosome aberrations may occur.
A number of studies have identified an increased rate of unstable chromosome aberrations such as dicentrics and rings in flight crewmembers, and related these to cosmic radiation exposure (
20,
21,
22). Nicholas et al. noted that unstable aberrations decrease with time and therefore do not serve as good indicators of cumulative exposure to
GCR. They postulate that structural chromosome aberrations such as translocations may be a better marker because they are relatively stable over time since exposure (
23). Nicholas et al. also showed that the mean number of translocations per cell was significantly higher among the airline pilots who were studied compared to controls. However, within the radiation exposure range encountered in the study, observed values among the pilots did not correspond to the dose-response pattern expected on the basis of available models for chronic low-dose radiation exposure. In addition, this study does not determine the role of radiation in the induction of translocations and so far, no epidemiologic evidence links these aberrations with the development of cancers.
Studies of chromosome aberrations with high-LET radiation, including heavy ions, show that the complexity of chromosome aberrations also increases with LET (
24). These studies are made using multicolor fluorescence
in-situ hybridization (FISH), where chromosome-specific probes are used to label individual chromosomes, and aberrations between two or more chromosomes are observed after irradiation as illustrated in
Figure 8-3. The number of chromosomes involved in chromosomal aberrations appears to increase with the LET of the radiation field. George et al. (
25) reported the number and types of chromosomal aberrations in astronauts on the International Space Station (
ISS).
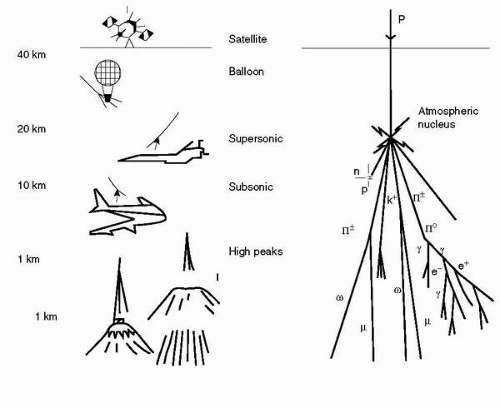
The biological effect of ionizing radiation depends upon whether it is high or low LET. Early studies of the effect of identical doses of different types of radiation on biological systems showed that different amounts of damage were produced. This led to the concept of “relative biological effectiveness” (RBE), which is defined as the ratio of a dose of a particular type of radiation to the dose of γ rays or x-rays that yield the same biological endpoint.
The dose equivalent to the tissue (DE) is the product of the absorbed dose (D) and the quality factor (Q or QF),
Q being dependent upon LET. The numerical value of
Q depends not only on appropriate biological data but also on the judgment of the ICRP. It establishes the value of the absorbed dose of any radiation that engenders the same risk as a given absorbed dose of a reference radiation (
26). The radiation weighting factor (
WR) takes account of quality factor, and recommendations are published from time to time by the ICRP (
26).
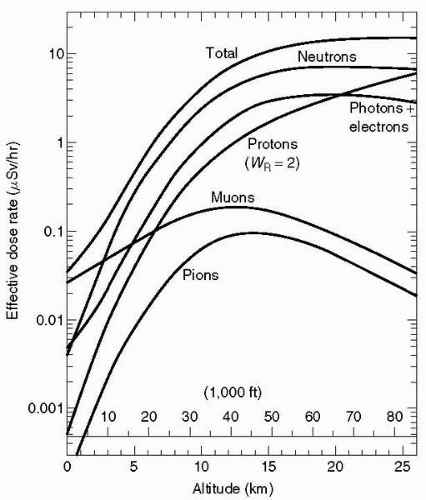
Low-LET radiation, all with a weighting factor of 1, includes photons, x- and γ rays, as well as electrons and muons. Electrons are the low-LET radiation of prime concern at aircraft operating altitudes.
Neutrons,
α-particles, fission fragments and heavy nuclei are classified as high LET, with neutrons providing approximately half the effective dose at high altitudes.
At all altitudes from 10,000 ft to more than 80,000 ft (3-25
km) neutrons are the dominant component of the cosmic radiation field. They are less dominant at lower latitudes, but still contribute 40% to 65% of the total dose equivalent rate. Because neutron interactions produce lowenergy ions, neutron radiation is more effective in inducing biological damage than
γ radiation. However, there are no adequate epidemiologic data to evaluate to what extent neutrons are carcinogenic to humans (
27).
The current weighting factors are shown in
Table 8-2. The weighting factor for neutrons depends on the energy of the incident neutrons. ICRP Publication 92 proposes that the means of computation of the factor should be a continuous function of energy rather than the step function given in Publication 60 (
26).
These proposals are based on current knowledge of biophysics and radiobiology, and acknowledge that judgments about these factors may change from time to time.
(ICRP recommends that no attempt be made to retrospectively correct individual historical estimates of effective dose or equivalent dose in a single tissue or organ. Rather the revised weighting factor should be applied from the date of adoption.)
Radiation Units of Measurement
The standard unit of radioactivity is the Becquerel (Bq), which is defined as the decay of one nucleus per second.
When considering cosmic radiation the practical interest is in the biological effect of a radiation dose, the dose equivalent being measured in Sievert (Sv). The ICRP has recommended a number of quantities based on weighting absorbed dose, to take account of the RBE of different types of radiation. Dose equivalent (Sv) is one of these.
Dose equivalent (H) is defined as:
H(LET) = Q(LET) × D(LET)
where Q is the quality factor and is a function of LET, and D is the absorbed dose.
The effective dose is obtained by the use of absorbed dose, D, along with different weighting factors for organs and tissues.
Doses of cosmic radiation are of such a level that values are usually quoted in microSievert (μSv) per hour or milli-Sievert (mSv) per year (1 mSv = 1,000 μSv).
The Sievert has superseded the rem as the unit of measurement of effective dose (1 Sv = 100 rem, 1 mSv = 100 mrem, 1 μSv = 0.1 mrem).
Other Terrestrial Sources of Ionizing Radiation
There is a constant background flux of ionizing radiation at ground level. Terrestrial background radiation from the Earth’s materials contributes 2.6 mSv/yr in the United Kingdom and 3 mSv/yr in the United States (
28). This flux is dominated by the low-LET component (93%).
Inhaled radon gas contributes approximately 2 mSv/yr to the total overall background ionizing radiation level (
28).
Medical x-rays are delivered in a concentrated localized manner, and usual doses are of the order (
28):
Chest x-ray |
0.1 mSv (100 μSv) |
Body computed tomographic (CT) scan |
10 mSv |
Chest CT scan |
8 mSv |
Intravenous pyelogram (IVP) |
1.6 mSv |
Mammogram |
0.7 mSv (700 μSv) |
These are effective doses averaged over the entire body, accounting for the relative sensitivities of the different tissues exposed.
Doses received from radiotherapy for cancer treatment range from 20 to 80 Sv (
29). These are all average figures with wide individual variations.