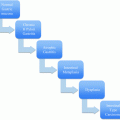
Fig. 1
Proposed algorithm for non-operative analysis with the gene expression classifier and somatic mutation testing in indeterminate thyroid nodules
Surgical Treatment of Thyroid Cancer
Patients with a diagnosis of DTC should undergo total thyroidectomy as recommended by the American Thyroid Association (ATA) [18]. Patients with medullary thyroid cancer receive total thyroidectomy with central neck lymph node dissection. In instances where the tumor is within a unifocal, intrathyroidal nodule, measuring <1 cm in dimension and having low-risk features, and in the absence of a history of head or neck irradiation or lymph node involvement, thyroid lobectomy may be the appropriate treatment. Similarly, patients with clinically or radiographically positive metastatic lymph nodes should undergo total thyroidectomy and concomitant ipsilateral, compartment-oriented lymph node dissection. Comprehensive preoperative neck ultrasound not only provides the opportunity for FNA biopsy of any suspicious nodes prior to surgery but also allows the surgeon to plan the appropriate surgery and counsel the patient regarding the surgery and its associated risks [19].
Cervical Lymph Node Dissection
The risk of regional recurrence of thyroid cancer historically ranges from 20 to 59 % and most frequently presents as nodal metastases. Indeed, these recurrences may actually be residual disease left behind at the time of initial surgery. The compartment-oriented central neck dissection extends from the hyoid bone superiorly to the innominate vein inferiorly; the medial border of the carotid sheath laterally and midline trachea medially. The central neck contents include the prelaryngeal, pretracheal, and paratracheal lymph nodes (Fig. 2).
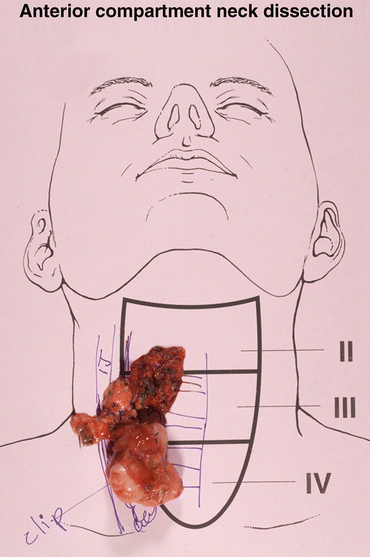

Fig. 2
Diagram of anatomical landmarks for compartment-oriented central neck lymph node dissection
In the lateral neck, a complete compartment-oriented modified lateral neck dissection should occur if nodal disease is present. This dissection should include the superficial layer of cervical fascia wrapping of the sternocleidomastoid muscle along with identification, preservation, and dissection of the spinal accessory nerve and vascular sheath (Fig. 3). The standard nodal draining pattern of involved disease does not usually extend higher than level II, so dissection in the submental region is not necessary. Intraoperative ultrasound can be performed to identify the suspicious-appearing lymph nodes above the posterior belly of the digastric.
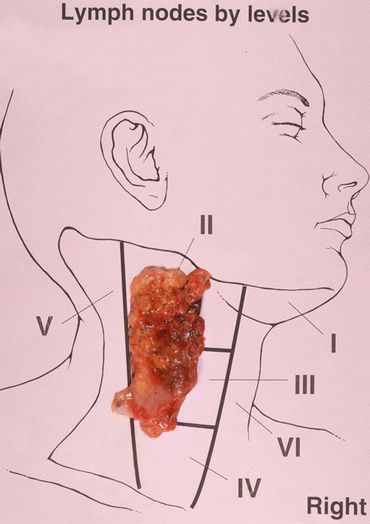

Fig. 3
Diagram of anatomical landmarks for compartment-oriented lateral neck lymph node dissection
Prophylactic Dissection vs. Therapeutic Dissection of the Central Compartment Lymph Nodes
The majority of surgeons would agree that patients with papillary thyroid cancer and radiographically, clinically, or intraoperatively suspicious or biopsy-proven metastatic lymph nodes warrant total thyroidectomy and compartment-based removal of the lymph node basin(s). Current controversy exists, however, as to the appropriate treatment of non-enlarged lymph nodes of the central neck at the time of initial thyroidectomy. Specifically, some groups advocate routine prophylactic central node dissection (PCND) for all patients with known papillary thyroid cancer to decrease the risk of local recurrence, even recognizing that no prospective randomized data support a survival benefit [20, 21]. While two retrospective studies have reported a reduction in disease recurrence rates associated with PCND [22, 23], two recent meta-analyses have shown that PCND does not reduce recurrence rates in a clinically significant manner [24, 25]. Moo et al. endorsed PCND, arguing that those patients noted to have pathologically positive nodal metastases after PCND were upstaged and therefore received more aggressive postoperative therapy with radioactive iodine ablation. However, no studies have actually shown an improvement in long-term survival with upstaging [21].
Surgery of the neck is not without complication. Surgeons arguing against PCND are unwilling to subject patients to the risks of recurrent laryngeal nerve (RLN) injury and permanent hypocalcemia by performing a procedure that has not yet clearly been demonstrated to improve long-term outcomes. Reoperation in the neck increases the risks of hypoparathyroidism and injury to the RLN, and these risks have been used as arguments in favor of PCND. For this reason, Popadich et al. studied the rate of central neck reoperation in patients undergoing PCND with total thyroidectomy and in those undergoing total thyroidectomy alone. These investigators demonstrated a significantly lower rate of reoperation in the central neck for those patients receiving PCND, but the overall rate of reoperation between the two groups was similar. They also noted a significantly higher rate of temporary hypocalcemia and transplantation of parathyroid glands in patients receiving PCND [26]. In their meta-analysis, Shan et al. reported the rate of temporary hypocalcemia to be significantly higher in patients undergoing PCND with thyroidectomy than in patients receiving thyroidectomy alone (31 % vs. 15 %, respectively, risk difference, 0.15; 95 % confidence interval, 0.09–0.22; P < 0.01). However, the definition of hypocalcemia did vary among the studies analyzed, and no difference among the studies was noted in the rate of permanent hypocalcemia. Shan and colleagues found that the incidence of transient RLN injury trended to be higher in patients undergoing PCND, but that increase did not reach statistical significance [24]. There is concern that these data underreported the risks of hypocalcemia and nerve injury because the studies were conducted in high-volume tertiary care centers where PCND is more commonly performed. Surgeons performing PCND less frequently may experience higher complication rates.
The current recommendations published by the ATA state that prophylactic or bilateral Level VI lymph node dissection is recommended in patients with T3/T4 papillary tumors but may not be necessary in patients with T1, T2, N0 thyroid cancers. The ATA also states that this recommendation should be interpreted in light of available surgical expertise, acknowledging that PCND may lead to increased perioperative morbidity [18]. Ideally, a prospective randomized trial would be conducted to help determine the benefit of PCND. The ATA evaluated the design and feasibility of such a study and concluded that the required sample size would be prohibitively large and therefore this study is not readily feasible [27]. Unfortunately, high quality evidence to determine the benefit of PCND is not available at the present time. Currently, selective, rather than routine, PCND seems the most reasonable option to guide the decision process. We have noted excellent long-term regional disease control and patient survival using preoperative US and intraoperative surgeon evaluation [28].
Postoperative Therapy
Radioactive Iodine Ablation
Adjuvant therapy for patients with DTC predominantly consists of radioactive iodine ablation (RAI) and thyroid-stimulating hormone (TSH) suppression. 131Iodine (131I) is used for therapeutic ablation of any remnant or microscopic thyroid tissue that may persist after surgery. For patients with high-risk disease, the use of RAI has been associated with reduced rates of recurrence and cause-specific mortality. RAI has not been shown to be beneficial in patients at low risk for disease recurrence or cause-specific mortality [29]. The decision to treat patients with RAI in the adjuvant setting is typically reserved for papillary or follicular thyroid cancer patients who are 45 years of age or older, patients whose primary tumor is >1 cm in diameter or multifocal (at least one nodule >1 cm in greatest dimension), and for patients with extrathyroidal disease due to tissue invasion. RAI may also be used in the treatment of local recurrence or distant metastases.
Complications associated with RAI include swelling and discomfort of the salivary glands, impaired sensation of taste (frequently metallic taste), dry mouth, nausea in the acute setting, pulmonary fibrosis, bone marrow suppression, and the potential for secondary cancers [30]. Given these potential side effects, practitioners are trending toward using lower initial doses of 131I (30–100 mCi) in patients with low-volume disease limited to the thyroid, and who have shown radioiodine uptake in only the thyroid bed on initial postoperative diagnostic whole-body scan [31]. Higher 131I doses are reserved for those patients with evidence of extrathyroidal disease extension, lymph node metastases or significant radioiodine uptake in the neck on initial postoperative diagnostic thyroid scan.
After total thyroidectomy and subsequent RAI (if indicated), patients will need lifelong thyroid hormone replacement. The levothyroxine sodium starting dose is 2 μg/kg/day and is then titrated to reach an appropriate level of TSH suppression. The amount of TSH suppression is also individualized based on the patient’s disease status and clinicopathological tumor features. Patients must also begin a surveillance regimen consisting of laboratory evaluation and ultrasonography. In papillary and follicular carcinoma, T4 and TSH demonstrate the level of thyroid suppression, and thyroglobulin and thyroglobulin antibody levels are important markers for possible disease recurrence or metastases. In medullary thyroid cancer, calcitonin and carcinoembryonic acid (CEA) levels should be monitored both pre- and postoperatively to identify trends suspicious for disease recurrence.
Systemic Therapy for Metastatic and RAI-Refractory Cancers
Patients with DTC generally have a good prognosis due to the indolent nature of the disease as well as to the efficacy of standard treatment. Even those with locoregional recurrence are typically treated with further surgery (Fig. 4) or RAI and have excellent outcomes. However, a subset of patients will develop locally recurrent disease but are no longer candidates for surgical resection. Metastatic disease also is observed in 15 % of patients with DTC, of whom half have metastatic disease diagnosed at the initial presentation. Patients with metastatic disease typically do not undergo further surgery as the benefits are only palliative in nature. RAI may be used to treat metastatic disease, but unfortunately, the majority of these patients will not experience complete remission despite multiple treatments [32, 33]. Further, RAI therapy is not indicated when tumors have proven resistant to RAI by evidence of radiographic progression, if tumors are non-avid on imaging, or if patients have experienced toxicity to RAI. Overall, these tumors carry a worse prognosis but may be stable and indolent over several years. Systemic therapy is reserved for progressive or symptomatic locally advanced or metastatic disease [34].
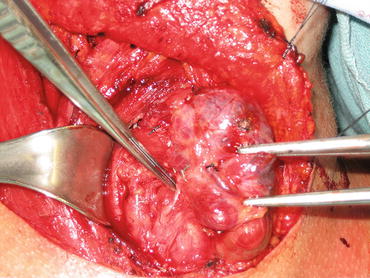

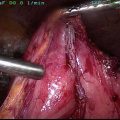
Fig. 4
Recurrent papillary thyroid cancer in the lymph nodes posterior to the right recurrent laryngeal nerve
Historically, cytotoxic chemotherapy, specifically doxorubicin, was used to treat metastatic thyroid cancers but produced a poor response. Systemic therapy is emerging in the form of antineoplastic therapies targeted at known gene mutations. Major targets involve inhibiting the kinase signaling pathways in tumor cells and vascular endothelial cells. Known mutations of the RET/PTC, BRAF, and RAS genes, vascular endothelial growth factor (VEGF) receptors, and epidermal growth factor (EGF) receptors and their downstream effects are of particular interest [33, 34]. Tyrosine kinase inhibitors (TKI) affect multiple signaling pathways and have shown promising results. The U.S. Food and Drug Administration (FDA) has approved the use of sorafenib for metastatic DTC and has approved vandetanib and cabozantinib for metastatic or unresectable MTC [35]. A systematic meta-analysis of eight phase II trials and retrospective studies evaluating the effects of sorafenib on disease progression, disease response and patient survival, found that sorafenib was associated with a partial response in 22 % of MTC patients, 21 % of DTC patients, and 13 % of anaplastic thyroid cancer patients. The majority of DTC and MTC patients demonstrated clinical benefit, with progression of disease noted in 6.5 % of MTC patients and 21 % of DTC patients [35].
A recent multicenter, prospective, randomized, double-blind, placebo-controlled, phase III trial entitled DECISION, was conducted to evaluate the efficacy and safety of sorafenib treatment in patients with RAI-refractory metastatic DTC. This trial included 417 patients, 207 of whom received sorafenib; however, the patients with disease progression in the placebo study arm were allowed to cross over to open-label sorafenib. The DECISION trial investigators reported a significantly longer progression-free survival in patients treated with sorafenib (10.8 months) than in patients in the placebo arm (5.8 months; hazard ratio, 0.59; 95 % confidence interval, 0.45–0.76; P < 0.0001). Adverse effects of sorafenib were noted in 98.6 % of treated patients and consisted mostly of the commonly reported side effects of hand–foot skin reaction, diarrhea, alopecia, and skin desquamation [36]. Of note, sorafenib has also been associated with the development of squamous cell carcinoma of the skin and therefore patients must be carefully monitored for skin cancer during and after treatment.
These promising studies have led not only to further research and development of targeted therapy in metastatic DTC, but also to expanded use of the currently approved therapies in the adjuvant setting. As more data accumulate, these therapies may be utilized in the neoadjuvant setting as well.
Special Considerations
Timing of Prophylactic Thyroidectomy for RET Mutation-Positive Patients
Unlike well-differentiated thyroid cancer, MTC is not derived from follicular cells but rather from calcitonin-producing C-cells of the thyroid. The development of MTC is sporadic in 80 % of patients; however, MTC is a distinct entity in the diagnosis of hereditary syndromes such as multiple endocrine neoplasia (MEN) type 2A and type 2B as well as familial medullary thyroid cancer (FMTC). The RET proto-oncogene mutation previously discussed is strongly associated with MTC and is present in 98 % of MEN 2A patients and in 95 % of FMTC individuals. Even those without an inherited etiology of MTC will frequently have a RET mutation identified upon genetic testing [37, 38]. Mutations in several different codons of the RET oncogene lead to varying degrees of transformation to MTC and to various phenotypes of the hereditary MTCs. For this reason, relatives of patients with a RET mutation should undergo genetic counseling and testing to detect carriers of mutations that may not yet have manifested clinical symptoms. Given its strong association with MTC, all family members identified to carry a RET gene mutation are recommended to undergo thyroidectomy. The timing of thyroidectomy for prophylactic, rather than therapeutic purposes in RET-mutation positive individuals is debatable.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree