Radiotherapy plays an integral role in the management of most patients with cancers of the head and neck. Better understanding of radiobiology and radiation physics has allowed radiation oncologists to enhance the tumoricidal effects of radiation and reduce the severity of normal tissue toxicities. This article reviews the biologic foundation of head and neck radiotherapy, the physical principles and technological innovations that enable delivery of highly conformal radiation, the acute and late complications of radiation-based treatments, and the clinical evidence supporting contemporary practice.
Key points
- •
Altered fractionation strategies improve outcome compared with conventional once-daily treatment of patients with locally advanced disease being treated with radiation therapy alone.
- •
Technological advances in diagnostic imaging and radiation delivery have improved the therapeutic ratio, with better disease delineation and sparing of normal tissues.
- •
Concurrent chemoradiation with intensity-modulated radiation therapy constitutes the standard nonsurgical therapy for locally advanced squamous cell head and neck cancer.
- •
Intensification strategies that add molecularly targeted agents to standard chemoradiation have resulted in increased toxicity but not improved efficacy to date.
- •
For patients who initially present with lymph node involvement, after chemoradiation PET-CT can distinguish those who do not require adjuvant neck dissection from those who do.
Radiation therapy: biologic basis and fractionation
Approximately 49,000 cases of head and neck squamous cell cancer (HNSCC) are diagnosed in the United States annually; nearly 60% present with locally advanced but nonmetastatic disease. Locoregional recurrence is the predominant pattern of failure from which most fatalities result. As a locoregional therapy, radiotherapy (RT) plays a primary role in treatment.
Ionizing radiation causes cytotoxic effects via free radical–mediated DNA damage. This process precipitates a cascade of physicochemical reactions that lead to single-strand and double-strand DNA breaks, loss of reproductive integrity, and cell death. In the earliest days of RT, treatments often involved single large doses (fractions) of radiation. Unfortunately, clinical responses were often accompanied by significant early and late toxicities. More protracted fractionated courses of RT were subsequently devised, which spread the treatment course out via the delivery of multiple smaller doses, with significantly reduced toxicity.
Four “Rs” underlying the radiobiology of fractionation have been described: repair of sublethal injury, cell repopulation, redistribution into more radiosensitive phases of the cell cycle, and reoxygenation. These concepts have influenced the way RT is delivered for all types of cancer, including HNSCC. Nonmalignant cells more efficiently repair radiation-induced DNA damage than their malignant counterparts. Therefore, multiple radiation fractions give normal tissues time to repair sublethal damage, preferentially killing tumor cells. However, longer overall treatment times promote repopulation of both normal and cancerous cells. Consequently, as fractionated regimens become more protracted, they typically require higher total doses to achieve similar degrees of tumor control. Nonetheless, the delivery of each dose within a fractionated regimen redistributes more cells into the more radiosensitive G2/mitosis phase of the cell cycle, which enhances radiation-induced cell kill. One final advantage of fractionation is that it promotes tumor reoxygenation. Hypoxic cells are 2.5 to 3 times more resistant to a given dose of radiation than their well-oxygenated counterparts. Tumor hypoxia adversely affects the prognosis of head and neck cancer.
Strategies to improve tumor control must be balanced against the ability of patients to tolerate the acute side effects of treatment and the potentially irreversible chronic toxicities that may subsequently occur. A conventional fractionation regimen used to treat HNSCC typically consists of once-daily 2 gray (Gy) fractions, five fractions per week, over 7 weeks, to a total dose of 70 Gy. This treatment is relatively well-tolerated with manageable mucositis; roughly one-third of patients experience some degree of grade 3 acute toxicity, and 2-year locoregional control (LRC) and overall survival (OS) rates are around 45%. Altered fractionation regimens, such as hyperfractionation and accelerated fractionation, represent radiobiology-based approaches to improve treatment efficacy.
Hyperfractionation is designed to deliver higher total doses of RT to improve disease control without increasing late toxicity relative to conventional fractionation. Pure hyperfractionation regimens keep the same overall treatment time as conventional therapy but achieve higher doses via delivery of smaller, multiple daily fractions. Smaller individual doses of RT allow for preferential repair of sublethal DNA damage in normal tissues that are responsible for the development of late side effects compared with tumor cells. A commonly used regimen is 1.2 Gy twice daily, ten fractions per week, over 7 weeks, to a total dose of 81.6 Gy.
Pure accelerated regimens deliver the same or slightly reduced total dose of RT in a shorter overall treatment time relative to conventional fractionation. The goal of the condensed timeframe is to overcome tumor repopulation, which accelerates 3 to 4 weeks after RT initiation. A typical regimen developed by the Danish Head and Neck Cancer Group (DAHANCA) uses 2 Gy fractions, six fractions per week, to a total dose of 70 Gy in 6 weeks. Hybrid regimens combine elements of hyperfractionation and acceleration. The concomitant boost regimen of the Radiation Therapy Oncology Group (RTOG) delivers a total dose of 72 Gy over 6 weeks, using 1.8 Gy daily fractions in the morning with the addition of a second 1.5 Gy fraction in the afternoon on the last 12 days of treatment to combat accelerated tumor repopulation.
Multiple trials have compared altered fractionation to conventional fractionation in locally advanced HNSCC. RTOG 9003 randomized 1073 subjects to standard 70 Gy versus pure hyperfractionation 81.6 Gy versus concomitant boost 72 Gy versus a split-course hybrid regimen of 67.2 Gy in 1.6 Gy twice-daily fractions over 6 weeks, with a scheduled 2-week break after 38.4 Gy. All three altered fractionation regimens caused significantly worse acute toxicity than standard RT but no increase in late effects. The hyperfractionated and concomitant boost arms had significantly better LRC than the standard arm with a trend toward improved disease-free survival (DFS). Overall survival was the same among the different arms. Subjects in the split course arm had control rates similar to the standard arm, suggesting that the treatment break had allowed for tumor repopulation. Altered fractionation studies published by the DAHANCA group (70 Gy in 6 weeks) and the European Organization for Research and Treatment of Cancer (EORTC; 1.15 Gy twice-daily to 80.5 Gy in 7 weeks) have shown similar improvements in LRC at the cost of increased but manageable acute toxicity.
Other trials have explored very accelerated fractionation regimens, delivering approximately 70 to 72 Gy over 5 weeks. These studies have shown improvements in LRC but at the cost of significantly higher rates of acute and late toxicities. Highly toxic regimens that require scheduled or unanticipated treatments breaks are self-defeating as prolongation to allow for repair and repopulation of normal tissues allows the same for cancer cells. Retrospective analyses have shown that extension of overall treatment time adversely affects local control, perhaps by 1% to 2% per day.
Altered fractionation regimens represent the successful translation of fundamental radiobiologic principles into clinical solutions that balance the dual goals of cancer control and treatment toxicity. A meta-analysis of individual patient data from 15 trials with 6515 patients showed an absolute 5-year LRC benefit of 6.4%, which translated into a 3.4% benefit in 5-year OS with altered fractionation strategies. These data provide the foundation for HNSCC management with RT as a single modality. However, treatment of locally advanced disease has evolved with a shift toward intensification with concurrent systemic agents.
Radiation therapy: biologic basis and fractionation
Approximately 49,000 cases of head and neck squamous cell cancer (HNSCC) are diagnosed in the United States annually; nearly 60% present with locally advanced but nonmetastatic disease. Locoregional recurrence is the predominant pattern of failure from which most fatalities result. As a locoregional therapy, radiotherapy (RT) plays a primary role in treatment.
Ionizing radiation causes cytotoxic effects via free radical–mediated DNA damage. This process precipitates a cascade of physicochemical reactions that lead to single-strand and double-strand DNA breaks, loss of reproductive integrity, and cell death. In the earliest days of RT, treatments often involved single large doses (fractions) of radiation. Unfortunately, clinical responses were often accompanied by significant early and late toxicities. More protracted fractionated courses of RT were subsequently devised, which spread the treatment course out via the delivery of multiple smaller doses, with significantly reduced toxicity.
Four “Rs” underlying the radiobiology of fractionation have been described: repair of sublethal injury, cell repopulation, redistribution into more radiosensitive phases of the cell cycle, and reoxygenation. These concepts have influenced the way RT is delivered for all types of cancer, including HNSCC. Nonmalignant cells more efficiently repair radiation-induced DNA damage than their malignant counterparts. Therefore, multiple radiation fractions give normal tissues time to repair sublethal damage, preferentially killing tumor cells. However, longer overall treatment times promote repopulation of both normal and cancerous cells. Consequently, as fractionated regimens become more protracted, they typically require higher total doses to achieve similar degrees of tumor control. Nonetheless, the delivery of each dose within a fractionated regimen redistributes more cells into the more radiosensitive G2/mitosis phase of the cell cycle, which enhances radiation-induced cell kill. One final advantage of fractionation is that it promotes tumor reoxygenation. Hypoxic cells are 2.5 to 3 times more resistant to a given dose of radiation than their well-oxygenated counterparts. Tumor hypoxia adversely affects the prognosis of head and neck cancer.
Strategies to improve tumor control must be balanced against the ability of patients to tolerate the acute side effects of treatment and the potentially irreversible chronic toxicities that may subsequently occur. A conventional fractionation regimen used to treat HNSCC typically consists of once-daily 2 gray (Gy) fractions, five fractions per week, over 7 weeks, to a total dose of 70 Gy. This treatment is relatively well-tolerated with manageable mucositis; roughly one-third of patients experience some degree of grade 3 acute toxicity, and 2-year locoregional control (LRC) and overall survival (OS) rates are around 45%. Altered fractionation regimens, such as hyperfractionation and accelerated fractionation, represent radiobiology-based approaches to improve treatment efficacy.
Hyperfractionation is designed to deliver higher total doses of RT to improve disease control without increasing late toxicity relative to conventional fractionation. Pure hyperfractionation regimens keep the same overall treatment time as conventional therapy but achieve higher doses via delivery of smaller, multiple daily fractions. Smaller individual doses of RT allow for preferential repair of sublethal DNA damage in normal tissues that are responsible for the development of late side effects compared with tumor cells. A commonly used regimen is 1.2 Gy twice daily, ten fractions per week, over 7 weeks, to a total dose of 81.6 Gy.
Pure accelerated regimens deliver the same or slightly reduced total dose of RT in a shorter overall treatment time relative to conventional fractionation. The goal of the condensed timeframe is to overcome tumor repopulation, which accelerates 3 to 4 weeks after RT initiation. A typical regimen developed by the Danish Head and Neck Cancer Group (DAHANCA) uses 2 Gy fractions, six fractions per week, to a total dose of 70 Gy in 6 weeks. Hybrid regimens combine elements of hyperfractionation and acceleration. The concomitant boost regimen of the Radiation Therapy Oncology Group (RTOG) delivers a total dose of 72 Gy over 6 weeks, using 1.8 Gy daily fractions in the morning with the addition of a second 1.5 Gy fraction in the afternoon on the last 12 days of treatment to combat accelerated tumor repopulation.
Multiple trials have compared altered fractionation to conventional fractionation in locally advanced HNSCC. RTOG 9003 randomized 1073 subjects to standard 70 Gy versus pure hyperfractionation 81.6 Gy versus concomitant boost 72 Gy versus a split-course hybrid regimen of 67.2 Gy in 1.6 Gy twice-daily fractions over 6 weeks, with a scheduled 2-week break after 38.4 Gy. All three altered fractionation regimens caused significantly worse acute toxicity than standard RT but no increase in late effects. The hyperfractionated and concomitant boost arms had significantly better LRC than the standard arm with a trend toward improved disease-free survival (DFS). Overall survival was the same among the different arms. Subjects in the split course arm had control rates similar to the standard arm, suggesting that the treatment break had allowed for tumor repopulation. Altered fractionation studies published by the DAHANCA group (70 Gy in 6 weeks) and the European Organization for Research and Treatment of Cancer (EORTC; 1.15 Gy twice-daily to 80.5 Gy in 7 weeks) have shown similar improvements in LRC at the cost of increased but manageable acute toxicity.
Other trials have explored very accelerated fractionation regimens, delivering approximately 70 to 72 Gy over 5 weeks. These studies have shown improvements in LRC but at the cost of significantly higher rates of acute and late toxicities. Highly toxic regimens that require scheduled or unanticipated treatments breaks are self-defeating as prolongation to allow for repair and repopulation of normal tissues allows the same for cancer cells. Retrospective analyses have shown that extension of overall treatment time adversely affects local control, perhaps by 1% to 2% per day.
Altered fractionation regimens represent the successful translation of fundamental radiobiologic principles into clinical solutions that balance the dual goals of cancer control and treatment toxicity. A meta-analysis of individual patient data from 15 trials with 6515 patients showed an absolute 5-year LRC benefit of 6.4%, which translated into a 3.4% benefit in 5-year OS with altered fractionation strategies. These data provide the foundation for HNSCC management with RT as a single modality. However, treatment of locally advanced disease has evolved with a shift toward intensification with concurrent systemic agents.
Treatment toxicity
Benefits of treatment intensification, whether through altered fractionation or combined modality therapy, come at the price of increased acute and late toxicities. The RTOG toxicity criteria define acute effects as those developing within 90 days from RT commencement and late toxicities after 90 days. The distinction is somewhat arbitrary because some late complications, such as chronic edema or necrosis of soft tissue or bone, may result from severe acute insults. The term consequential late effect is often used to describe this phenomenon.
Common acute toxicities include mucositis, dermatitis, taste alteration, dry mouth, anorexia, and fatigue. Radiation-induced mucositis is the most significant of these and can cause significant pain and distress; it is usually the limiting factor to therapeutic intensification. Decreases in oral fluid and nutritional intake may require treatment breaks for intensive supportive care, including feeding tube placement.
Pathologic examination of inflamed mucosa reveals shallow ulcerations caused by depletion of the epithelial stem cell layer with denudation and subsequent formation of raised pseudomembranes composed of inflammatory cells, interstitial exudate, fibrin, and cell debris. Deep ulceration requiring prolonged healing may lead to consequential late mucosal effects. Some ulcers never heal and may result in soft tissue or bone necrosis. Attempts to mitigate RT-induced oral mucositis have focused on the use of palifermin, a recombinant human keratinocyte growth factor. In two phase III trials, palifermin reduced the incidence of observed grade 3 to 4 mucositis but without differences in subject-reported pain scores, narcotic usage, or duration of treatment breaks. Its role in HNSCC management remains investigational.
Potential late effects from HNSCC RT include chronic xerostomia, dysphagia, osteoradionecrosis, fibrosis, trismus, atrophy, hypothyroidism, brachial plexopathy, and carotid artery injury. Development of late complications is multifactorial with contributions from both patient-specific and treatment-related characteristics including age, inherent normal tissue radiosensitivity, primary tumor location and size, RT fraction size, total dose, irradiated volume, and the inclusion of other treatments such as surgery and/or chemotherapy.
Compared to definitive RT alone, surgery and postoperative RT resulted in significantly higher rates of late subcutaneous fibrosis on retrospective review of two prospective Trans Tasman Radiation Oncology Group (TROG) trials. The study compared 172 patients who received definitive RT (59.4 Gy, 1.8 Gy twice-daily) versus 52 patients who received surgery and postoperative RT (50.4–54 Gy, 1.8 Gy twice-daily). Despite receiving lower radiation doses, postoperative patients had significantly higher rates of subcutaneous fibrosis (34% vs 16%, P <.01). Review of three prospective RTOG trials showed a 43% incidence of severe (≥grade 3) late pharyngeal or laryngeal toxicity following concurrent chemoradiation (CRT). Multivariable analysis found that older age, advanced tumor (T) stage, location of disease in the larynx or hypopharynx, and neck dissection after CRT correlated significantly with the development of severe late toxicity. A similar impact of posttherapy neck dissection on late dysphagia was reported by Fox Chase Cancer Center, with relative risk (RR) of feeding tube dependence significantly higher at 18 months (RR 4.7) and 24 months (RR 7.7). These retrospective findings are hypothesis-generating only, but they inform the evolving role of adjuvant neck dissection following definitive RT or CRT.
The most common late complication in HNSCC patients treated with RT is xerostomia. Chronic dry mouth due to permanent damage to salivary glands has significant impact on patient quality of life causing difficulties with chewing and swallowing, disturbances in speech and sleep, and increased susceptibility to oral infections, dental caries, tooth loss, and osteonecrosis. Pharmacologic attempts to reduce the incidence of RT-induced xerostomia have centered on radioprotector compounds such as amifostine. A thiol-containing prodrug, amifostine preferentially accumulates in the kidneys and salivary glands where it is subsequently metabolized into an active free radical scavenger. In a phase III trial, the addition of amifostine to conventional RT alone significantly reduced the incidence of greater than or equal to grade 2 acute xerostomia (78% vs 51%, P <.001) and greater than or equal to grade 2 chronic xerostomia 1 year post-RT (57% vs 34%, P = .002). The beneficial effects of amifostine on duration and severity of xerostomia persisted 2 years posttreatment without compromising disease control or OS. Side effects of amifostine, including nausea or vomiting and hypotension, developed in nearly two-thirds of subjects, but less than 10% experienced greater than or equal to grade 3 toxicity. Still, 21% discontinued amifostine before the end of RT.
Amifostine was tested in patients treated with conventionally fractionated RT alone using nonconformal techniques that did not spare the parotid glands. Its efficacy in those treated with CRT and/or with modern parotid-sparing RT techniques, such as intensity-modulated RT (IMRT), is unclear. Small phase II-III trials suggest a benefit in the setting of CRT, but level I evidence is lacking. Given its side-effect profile, the widespread adoption of IMRT, and the increasing use of CRT, amifostine has not established a significant role in current HNSCC management.
Despite increased late toxicities with multimodality approaches, the worst possible complication remains uncontrolled disease. Therefore, intensive treatment strategies that maximize tumor control should be offered to suitable patients willing and able to tolerate therapy. Honest discussions between providers and patients allow for establishment of reasonable goals and realistic expectations of therapy.
Treatment planning and delivery
Minimizing unnecessary irradiation of adjacent tissues while maximizing dose delivery to tumor represents a fundamental and effective strategy to reduce complications. Advances in imaging technology and treatment delivery have revolutionized the ability to visualize deep structures and design treatments where the regions of high-dose delivery conform closely to target volumes. CT, MRI, and positron-emitting tomography (PET) provide complementary anatomic and functional information for radiation treatment planning.
The typical planning process for external beam RT in HNSCC requires: (1) reproducible immobilization with a thermoplastic mask; (2) simulation CT scan, preferably with intravenous contrast enhancement; (3) export of resulting CT images into a treatment planning software system with registration/fusion of other available imaging datasets (MRI, PET-CT) as clinically indicated; (4) delineation/contouring of all target volumes and normal tissue structures; (5) determination of the optimal number of beams and their respective angles and apertures; (6) generation and optimization of the treatment plan; (7) critical plan evaluation to ensure appropriate target coverage and normal tissue sparing; and (8) plan review and confirmation via quality assurance before treatment delivery.
Target delineation and designation are essential components of RT planning ( Fig. 1 ). Gross tumor volume (GTV) corresponds to clinically and/or radiographically apparent disease. The clinical target volume (CTV) encompasses GTV and areas of suspected subclinical, microscopic involvement, including regional nodal basins. Planning target volume (PTV) includes the CTV plus 3 to 5 mm margin to account for anatomic motion and variability in patient or beam setup.

Complex target volumes are common in head and neck cancers, and concave PTVs frequently abut organs such as the parotid glands, pharyngeal constrictor muscles, optic structures, and spinal cord. IMRT has revolutionized the treatment of HNSCC because it is capable of generating very conformal dose distributions around irregularly shaped tumors with steep dose fall-off immediately beyond target boundaries ( Fig. 2 ). Unlike conventional radiation techniques, which use static field apertures of uniform beam intensities, IMRT subdivides each beam into thousands of smaller beamlets of varying intensities. The intensity of each beamlet is determined iteratively via computer to generate treatment plans that meet prespecified dose parameters. A typical IMRT treatment plan is designed to ensure that greater than or equal to 95% of the PTV receives the prescribed dose, whereas the maximum dose to the spinal cord with margin is less than 45 to 50 Gy, and the mean dose to at least one parotid gland is less than 26 Gy.
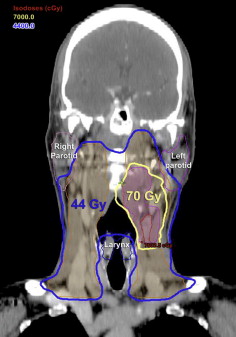
Lower doses to the parotids allow for better recovery of salivary function and less xerostomia. Quantitative scintigraphy studies have found IMRT considerably more effective than amifostine in protection of salivary gland function. Two small phase III trials from Hong Kong randomized subjects with nasopharyngeal cancer to IMRT or conventional RT. The use of IMRT was associated with lower rates of late xerostomia, greater recovery of salivary flow, and higher quality of life scores.
The PARSPORT study, a multicenter phase III trial from the United Kingdom, randomized 94 subects with oropharyngeal or hypopharyngeal squamous cell carcinomas to IMRT or conventional RT. Contralateral mean parotid doses were 22.4 Gy with IMRT versus 60 Gy with conventional RT ( P <.0003). Greater than or equal to grade 2 xerostomia, as assessed by the Late Effects of Normal Tissue (LENT SOMA) scale, was significantly lower with IMRT at 12 months (38% vs 74%) and 24 months (29% vs 83%). Subjects treated with IMRT also had greater objective recovery in salivary secretion and clinically significant improvements in dry-mouth–specific and global quality of life scores. No differences were noted in LRC or OS.
One essential tool radiation oncologists use to determine whether a treatment plan achieves the desired dose specifications is the cumulative dose-volume histogram (DVH). The radiation dose to a given target or normal structure is plotted on the X axis and percentage of the volume of that structure is plotted on the Y axis. Each point on the curve represents the percent volume of a particular structure receiving at least the indicated dose. For example, the DVH in Fig. 3 represents an IMRT plan in which 97% of the mandible is receiving at least 10 Gy, 87% is receiving at least 30 Gy, and 34% is receiving at least 50 Gy. In essence, DVH plots are designed to graphically display entire dose distributions for target volumes and normal tissue structures in a comprehensible format. The responsibility of the radiation oncologist is to recognize the clinically relevant dose-volume relationships for each normal tissue structure and understand their associated complication probabilities.
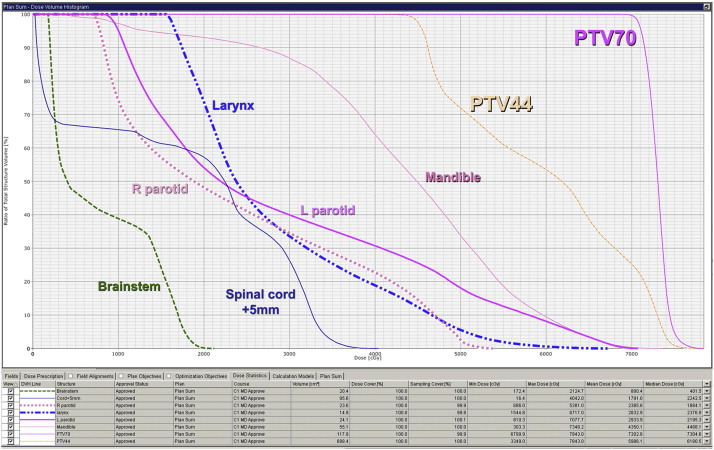
Different normal tissues require different dose and/or volume constraints. For example, radiation beyond tolerance to any segment of the spinal cord may result in permanent damage and myelopathy. Therefore, an acceptable RT plan may restrict the maximum dose to the cord below 45 to 50 Gy to minimize the risk of catastrophic paralysis, even at the expense of target volume coverage. Other organs function in a more parallel fashion and entire sections may be removed or destroyed with the remaining subunits still performing the necessary functions. For these organs, such as the parotid gland, a mean dose constraint more accurately reflects the risk of toxicity development than a maximum dose constraint.
Principles of therapy
Radiation Therapy Doses
For head and neck cancers, greater numbers of tumor clonogens require higher RT doses for eradication. Consequently, radiation dose prescriptions are usually made according to the disease burden with higher doses being delivered to regions of gross disease versus subclinical microscopic disease ( Table 1 ). Doses of 45 to 50 Gy given with 1.8 to 2 Gy fractions are typically used for areas at risk of harboring microscopic disease, such as elective nodal basins. Regions with larger but still subclinical deposits of disease, such as positive margins or extracapsular extension (ECE), require doses of 60 to 66 Gy. Finally, areas of gross disease receive greater than or equal to 70 Gy. Of note, the dose levels for elective volumes are sufficient to cause permanent xerostomia without careful planning. Also, for patients with nodal disease with clinical and/or radiographic signs of ECE, an upfront neck dissection in lieu of definitive CRT results in only minimal radiation dose reduction from 70 Gy to 66 Gy with concurrent chemotherapy and also exposes the patient to a higher risk of chronic toxicity from trimodality therapy that often is not necessary.