Consultative Hematology
![]()
HEMATOLOGIC COMPLICATIONS OF PREGNANCY
Anemia in Pregnancy
During normal pregnancies, plasma volume increases by 40% to 60% and red cell mass by 20% to 40%. The hematocrit typically decreases 30% to 32%, and the lower limit of normal for hemoglobin declines to 11 g/dL in the first trimester and 10 g/dL in the second and third trimesters. The most common forms of anemia of pregnancy in North America are due to iron and folate deficiencies.
One thousand milligrams of additional iron are required during pregnancy. The normal 500 mg iron storage pool is insufficient, and iron deficiency anemia develops in the absence of iron supplementation throughout pregnancy. The recommended daily allowance for iron during pregnancy is 27 mg of elemental iron. The United States Centers for Disease Control recommends routine low-dose iron supplementation (30 mg of elemental iron daily) for all pregnant women, beginning at the first prenatal visit. Calculations of dosage for iron preparations should be based on the amount of iron in each preparation: Ferrous sulfate contains 20% elemental iron, 12% ferrous gluconate, and 33% ferrous fumarate. Low values of serum iron and ferritin are reliable indicators of iron deficiency in pregnancy. The consequences of maternal iron deficiency on the neonate are controversial. Mild-to-moderate maternal iron deficiency anemia is not associated with significant anemia in the fetus.
Folate needs are increased during pregnancy. Folate deficiency is associated with anemia, neural tube defects, and cleft palate. Neural tube closure occurs during the fourth week of pregnancy; therefore, folate supplementation is necessary prior to conception to prevent neural tube defects. Most prenatal vitamins contain both folate and iron.
Sickle Cell Disease in Pregnancy
Women with sickle cell anemia are in a high-risk pregnancy group. With modern obstetric and perinatal care, maternal mortality is less than 1% and perinatal mortality is less than 15%.
Prophylactic red cell transfusions are associated with fewer maternal painful episodes, but they have no impact on maternal morbidity, infant birth weight, gestational age, fetal distress, or perinatal mortality.
Maintenance transfusions should be administered to women who are symptomatic of vasoocclusive or anemia-related problems or when signs of fetal distress are present.
Platelet count decreases by approximately 10% during pregnancy, mostly in the third trimester.
The most common cause of thrombocytopenia is incidental thrombocytopenia of pregnancy (75%), followed by thrombocytopenia complicating hypertensive disorders of pregnancy (20%) and finally immunological disorders of pregnancy (5%).
Thrombocytopenia of less than 100,000/μL in the first trimester of pregnancy is most typical for immune thrombocytopenic purpura. Thrombocytopenia of over 70,000/μL occurring late during the second trimester or during the third trimester, in the absence of hypertension or proteinuria, usually represents incidental thrombocytopenia of pregnancy. Platelet-associated IgG is elevated in both incidental thrombocytopenia of pregnancy and immune thrombocytopenic purpura.
It is important in any patient with thrombocytopenia to consider human immunodeficiency virus infection, systemic lupus erythematosus, and thrombocytopenia associated with antiphospholipid antibodies in the differential diagnosis.
Incidental Thrombocytopenia of Pregnancy
The platelet count in incidental thrombocytopenia generally remains above 100,000/μL. Incidental thrombocytopenia usually develops in the third trimester and is not associated with neonatal thrombocytopenia. The likelihood of a more serious cause of thrombocytopenia increases when the platelet count drops below 70,000/μL. The pathogenesis of incidental thrombocytopenia is not clearly defined but may involve a combination of hemodilution and decreased platelet half-life.
Incidental thrombocytopenia remains a diagnosis of exclusion. The diagnosis is made by the lack of other physical or laboratory abnormalities in patients who do not have an antecedent history of immune thrombocytopenia. Women with incidental thrombocytopenia should receive standard obstetrical care. A platelet count greater than 80,000/μL is felt to be sufficient for epidural anesthesia.
Immune Thrombocytopenic Purpura
Immune thrombocytopenia (ITP) is the most common cause of severe thrombocytopenia in the first trimester of pregnancy. An antecedent history of ITP or autoimmune disorder makes the diagnosis more likely. The nadir platelet count in ITP usually occurs in the third trimester.
Patients with platelet counts greater than 20,000 to 30,000/μL and no evidence of bruising or mucosal bleeding generally do not require treatment in the first two trimesters of pregnancy. A platelet count of greater than 50,000/μL is considered safe for normal vaginal delivery or cesarean section. Although there is no consensus, a platelet count greater than 80,000/μL is sufficient for epidural anesthesia. The bleeding time is not an accurate predictor of risk of bleeding in these situations.
Optimal first-line therapy for ITP in pregnant patients is controversial. Corticosteroids are the least expensive option, but they have been associated with pregnancy-induced hypertension, gestational diabetes, osteoporosis, excessive weight gain, and premature rupture of fetal membranes. The placenta metabolizes 90% of the administered dose of prednisone, and thus serious fetal side effects are unlikely. Prednisone is initiated at a dose of 1 mg/kg/day (based on the pre-pregnancy weight) and subsequently tapered to the minimum hemostatically effective dose. Intravenous immunoglobulin (IVIg) should be considered if the maintenance dose of prednisone is in excess of 10 mg/day. IVIg given at a dose of 1 g/kg (based on pre-pregnancy weight) is associated with a response in over 60% of patients, and response duration averages a month.
In patients refractory to corticosteroids and IVIg, splenectomy should be considered. Splenectomy is best performed in the second trimester of pregnancy. Splenectomy in the first trimester may induce labor, and splenectomy in the third trimester may be technically difficult. Splenectomy has been successfully performed laparoscopically during pregnancy. High-dose methylprednisolone and intravenous anti-D immunoglobulin have been used in small series of refractory patients.
Very little data are available regarding the safety and efficacy during pregnancy of thrombopoietin receptor mimetic agonists (romiplostim, eltrombopag). Experience with immunosuppressive and cytotoxic agents during pregnancy also is limited. Danazol and vinca alkaloids are best avoided. Interventions that raise maternal platelet count are not effective in augmenting the platelet count of the fetus.
The use of non-steroidal anti-inflammatory drugs should be avoided postpartum in patients with platelet counts less than 100,000/μL. Thromboprophylaxis should be considered in all women with a platelet count greater than 50,000/μL; if they have undergone surgical delivery, are immobilized for a prolonged amount of time, or have acquired or congenital thrombophilia.
Neonatal mortality is less than 1% in ITP. Five percent of neonates will have a platelet count of less than 20,000/μL; most hemorrhagic events in neonates occur 24 to 48 hours after delivery, at the nadir of the platelet count. There is no evidence that cesarean section is safer for the neonate than is vaginal delivery. The mode of delivery should be decided on the basis of routine obstetric indications.
Maternal platelet count, maternal platelet antibody levels, or a history of maternal splenectomy for ITP are not accurate predictors of neonatal platelet counts. The most accurate predictor of fetal thrombocytopenia is a history of thrombocytopenia at delivery in a prior sibling. Fetal scalp blood sampling and cordocentesis have been generally abandoned.
A cord platelet count should be determined following delivery in every neonate. Thrombocytopenic neonates should be followed closely following delivery, the platelet count nadir may not occur before 2 to 5 days. Neonates presenting with clinical bleeding or a platelet count less than 20,000 μL should be managed using IVIg at a dose of 1 g/kg. Life threatening bleeding can be treated with a combination of IVIg and platelet transfusions. Neonates with platelet counts less than 50,000/μL should undergo transcranial ultrasound to exclude intracranial hemorrhage.
Preeclampsia and HELLP Syndrome
Preeclampsia is defined as hypertension (systolic pressure greater than 140 mm of mercury or diastolic pressure greater than 90 mm of mercury) and proteinuria (greater than 300 mg of protein/24 hours) occurring after 20 weeks of gestation. Preeclampsia occurs in 5% of all pregnancies, and it accounts for 18% of maternal deaths in the United States. Predisposing factors include age below 20 or over 30, increased body mass index, chronic hypertension, and insulin resistance. Thrombocytopenia develops in 50% of patients with preeclampsia. Endothelial damage and activation of the coagulation system with thrombin generation may explain the thrombocytopenia. D-dimers and thrombin–anti-thrombin complexes are increased in patients with thrombocytopenia.
The criteria for HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) are as follows:
![]() microangiopathic hemolytic anemia
microangiopathic hemolytic anemia
![]() increased transaminases
increased transaminases
![]() lactic dehydrogenase greater than 600 units per milliliter
lactic dehydrogenase greater than 600 units per milliliter
![]() thrombocytopenia (less than 100,000/μL).
thrombocytopenia (less than 100,000/μL).
HELLP occurs in up to 10% of women with severe preeclampsia. Proteinuria is present in 75% of patients with HELLP syndrome, but only 50% to 60% have hypertension. The syndrome usually occurs in white, multiparous women above the age of 25 years. Maternal mortality is 1% and fetal mortality is 10% to 20%. Fetal mortality is attributed to placental ischemia, abruption of the placenta, immaturity, and intrauterine asphyxia. Neonatal thrombocytopenia can occur in both preeclampsia and HELLP. The mechanism of neonatal thrombocytopenia remains unclear. There is a 3% risk of recurrence of HELLP in subsequent pregnancies.
The definitive treatment for eclampsia and HELLP is delivery of the fetus. Management focuses on stabilization of the patient and maturation of the fetal lung. The presence of multiorgan dysfunction, fetal distress, or a gestational age greater than 34 weeks warrants immediate delivery. Coagulopathy resulting from preeclampsia-associated diffuse intravascular coagulation (DIC) occurs in 20% of patients. The clinical manifestations of preeclampsia and HELLP resolve within a few days of delivery. Rarely, HELLP syndrome can present postpartum. If the manifestations worsen or persist after 1 or 2 days, plasma exchange is indicated.
Acute fatty liver of pregnancy occurs in the third trimester and is associated with hypertension and proteinuria in 50% of patients. Microangiopathic hemolytic anemia and thrombocytopenia are not prominent in this syndrome. Patients usually have a prolonged prothrombin time, a low fibrinogen, and low antithrombin levels.
Thrombotic Thrombocytopenic Purpura and Hemolytic Uremic Syndrome
Thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS) occur in only 0.004% of pregnancies. The following are the classic pentad of symptoms of TTP:
![]() Microangiopathic hemolytic anemia
Microangiopathic hemolytic anemia
![]() Thrombocytopenia
Thrombocytopenia
![]() Neurologic abnormalities
Neurologic abnormalities
![]() Fever
Fever
![]() Renal dysfunction
Renal dysfunction
However, the classic pentad is present in only 40% of patients. Pregnancy is a precipitating factor for TTP. The mean time of onset of TTP is at 23.5 weeks of pregnancy. Plasma exchange therapy is recommended for the management of the pregnant TTP patient, and delivery is indicated only for patients who do not respond to plasma exchange. Pregnancy termination is not considered therapeutic in TTP or HUS.
Ultralarge von Willebrand factor (VWF) multimers are found in TTP, thought to be secondary to the deficiency of a specific VWF-cleaving protease, identified as ADAMTS-13. ADAMTS-13 levels in TTP are typically less than 10%. ADAMTS-13 deficiency can be congenital or acquired. Acquired deficiency is associated with the presence of autoantibodies directed against ADAMTS-13. Reduced ADAMTS-13 levels are not specific for TTP; reduced levels are seen in the third trimester of pregnancy, in uremia, acute inflammation, malignancy, and in DIC. The majority of patients with TTP respond to plasma exchange. The role of corticosteroids remains controversial, mainly because of associated side effects. Patients who develop pregnancy-associated TTP are at high risk of recurrence with subsequent pregnancies.
The mean time of onset of HUS is 26 days following delivery. Patients with HUS present with microangiopathic hemolytic anemia and acute renal failure. VWF levels are usually elevated while multimer analysis may or may not show ultra-large multimers. Deficiency of VWF—cleaving protease is usually not associated with this syndrome.
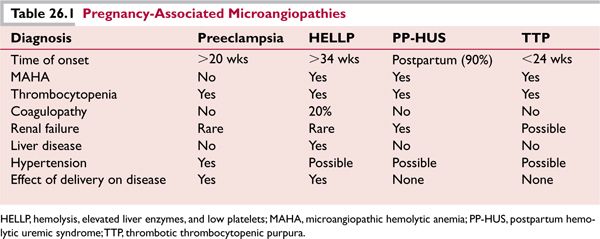
Several women with a familial history of pregnancy-associated HUS have developed their first episode of HUS during pregnancy, and HUS has occurred in such patients with the use of oral contraceptives. Postpartum HUS is associated with a poor prognosis. Plasma exchange is less effective in reversing renal failure in pregnancy-associated HUS. Nevertheless, a trial of plasma exchange is indicated. Dialysis and other supportive-care measures may also need to be initiated (Table 26.1).
Diffuse Intravascular Coagulation
Placental abruption is the most common cause of DIC (Table 26.2). There is an increased incidence of placental abruption in cocaine addicts. The incidence of DIC complicating placental abruption and dead fetus syndrome has decreased with advances in ultrasonography and prenatal care.
Placental abruption
Fetal death syndrome
Amniotic fluid embolism
HELLP syndrome
Clostridial sepsis
Sepsis
Major obstetrical hemorrhage
HELLP, hemolysis, elevated liver enzymes, and low platelets.
Fetal death syndrome is recognized by ultrasonography. Delivery of the dead fetus removes the source of tissue thromboplastin release. Blood component support and the use of antithrombin-3 have been useful in the management of the coagulopathy.
Placental abruption is managed with blood component support followed by delivery. Antithrombin-3 and activated protein C have been used with success in this disorder.
Transient DIC occurs in patients undergoing hypertonic saline abortions, but the DIC usually resolves once the fetus is delivered. Clostridial sepsis following abortions is associated with DIC and poor clinical outcome.
Venous Thromboembolism in Pregnancy
The per-day risk of venous thromboembolism (VTE) is increased 7- to 10-fold for antepartum VTE and 15- to 35-fold for postpartum VTE. The risk of VTE diminishes rapidly after delivery, returning to the antepartum risk level by 3 weeks postpartum and to the non-pregnant level after 6 weeks. Venous thrombi occur predominantly in the left leg, partly because of the compression of the left iliac vein by the right iliac artery as they cross.
Hemodynamic changes causing venous stasis and hypercoagulability play a role in the increased risk of VTE during pregnancy. Hypercoagulability is thought to be secondary to an increase in fibrinogen, factor VIII, and von Willebrand factor. Further, a decrease in protein S, the development of acquired protein C resistance, and reduced fibrinolytic activity from increased plasminogen activator inhibitor type 1 and 2 activity and decreased tissue plasminogen activator activity may contribute.
History of prior VTE, body mass index greater than 25, prolonged immobilization, inherited thrombophilias, antiphospholipid antibodies, and a family history of thrombosis all increase the risk of VTE during pregnancy.
Diagnosis of Venous Thromboembolism in Pregnancy
The diagnosis of VTE during pregnancy is complicated by the potential for fetal oncogenicity and teratogenicity due to ionizing radiation in diagnostic purposes.
Compression ultrasonography (CU) of the entire proximal venous system to the trifurcation should be performed as the initial test for suspected deep vein thrombosis (DVT) in pregnancy. A normal CU does not exclude a calf DVT. The CU needs to be repeated at day 2 and day 7 to exclude an extending calf-vein thrombosis. A limited venogram with fetal shielding can be used in equivocal cases. When iliac DVT is suspected, pulsed Doppler ultrasound should be used; if the results are negative or equivocal, magnetic resonance venography (MRV) or venography should be considered.
In patients with suspected pulmonary emboli during pregnancy, bilateral compression lower extremity ultrasounds should be performed. If the ultrasound is negative a ventilation/perfusion lung scan (V/Q) should be the next procedure. If the results of the V/Q scan are equivocal, computed tomography pulmonary angiography (CTPA) should be performed. However, should an isolated subsegmental defect be suggested by CTPA, additional testing is suggested because of the high false-positive rate.
D-dimer levels increase throughout pregnancy. The D-dimer test has a high sensitivity, relatively low specificity and very high negative predictive value.
Treatment of Venous Thromboembolic Disease in Pregnancy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



