Consultations in Anticoagulation
This chapter provides guidelines for the treatment of venous thromboembolism (VTE) in patients who require special consideration, such as those who have underlying cancer, malignancy, or who are pregnant. We also discuss use of inferior vena cava (IVC) filters, the prevention, diagnosis, and treatment of postthrombotic syndrome, and anticoagulant drugs with novel mechanisms that are now in development.
![]()
PROPHYLAXIS AND TREATMENT OF VENOUS THROMBOEMBOLISM IN THE PATIENT WITH CANCER IN SPECIFIC CLINICAL SETTINGS
Patients with malignancy have an increased risk for VTE due to multiple factors: hypercoagulability resulting from increased production and release of microparticles containing procoagulants such as tissue factor; vessel wall damage, impaired blood flow (stasis) from extrinsic compression; prolonged immobility, anticancer therapy including cytotoxic chemotherapy, certain antiangiogenic agents, or hormonal therapy, and the increasing use of long-term indwelling devices, such as central venous catheters. Tumor angiogenesis, progression, growth, and the metastatic process are enhanced by, and depend on activation of blood coagulation. P-selectin, a cell adhesion molecule has also been identified as a risk factor for recurrent VTE and can be used as a predictive parameter for development of VTE in cancer patients.1 As long as the cancer is active, the increased risk for VTE is present. Cancers most commonly associated with VTE are carcinomas of the pancreas, stomach, kidney, lung, ovary, and bladder, certain hematologic malignancies, cancers of the testis, and gliomas of the brain. Adenocarcinomas appear to be associated with a higher risk than squamous cell cancers.
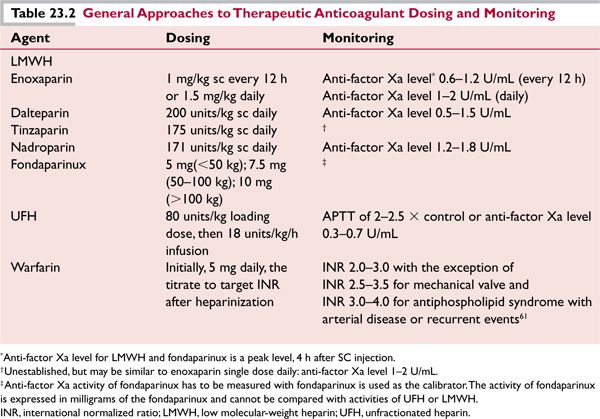
Studies performed during the current decade have demonstrated that low-molecular-weight heparin (LMWH) is more effective than oral anticoagulants in reducing the risk of recurrent VTE without increasing the risk of bleeding in patients with cancer and acute VTE. Products such as dalteparin, enoxaparin, nadroparin, and tinzaparin as well as the synthetic factor Xa inhibitor, fondaparinux are approved by the Food and Drug Administration (FDA) for the prophylaxis and treatment of VTE. In general, unfractionated heparin (UFH), LMWH, fondaparinux, and oral anticoagulants are the mainstay of therapy. Since LMWH undergoes renal excretion, patients with kidney impairment who receive LMWH should be monitored by measurement of the anti-factor Xa activity. The creatinine clearance should be estimated (or calculated) before initiation of LMWH in elderly patients, since they may have renal dysfunction despite having normal creatinine values. Specific dosing recommendations for enoxaparin in patients with severe renal insufficiency and/or low body weight are presented in Table 23.1. Monitoring of anti-factor Xa levels should also be used in severely obese patients (BMI ≥ 40) who receive therapeutic doses of LMWH and should be considered for those who are obese (BMI ≥ 30), especially if the patient has moderate to severe renal insufficiency (creatinine clearance less than 60 mL/min).2
The target peak anti-factor Xa levels (measured 4 hours after injection) for patients who are treated with LMWH vary according to the product. The target therapeutic range for enoxaparin is 0.6 to 1.2 U/mL for twice-daily dosing and 1 to 2 U/mL for once-daily dosing; for dalteparin the target range is 0.5 to 1.5 U/mL (Table 23.2). Since fondaparinux is produced by complete chemical synthesis and its structure is completely defined, it is dosed on the basis of mass rather than anti-factor Xa activity. The assay for fondaparinux is often reported in terms of its concentration in mass/volume (e.g., mg/L).
Primary Prophylaxis in Cancer Patients Undergoing Surgical Intervention
VTE is a common complication of cancer surgery and the most common cause of death at 30 days after surgery in the @RISTOS prospective observational study of cancer surgery patients.3 Prophylaxis should now be provided routinely to postoperative surgical patients, especially those with underlying cancer. Two randomized controlled trials have demonstrated that extending deep venous thrombosis (DVT) prophylaxis from 1 to 4 weeks reduces the incidence of VTE.4,5 Extended (up to 4 weeks) VTE prophylaxis is recommended for high-risk cancer surgery patients with a previous episode of VTE, anesthesia times longer than 2 hours, advanced stage disease, perioperative bed rest ≥4 days, and patient age ≥ 60 years.3
Laparoscopic surgery is rapidly becoming a common method for tumor resection. It is unclear how the recommendations developed for typical, open procedures should be applied to laparoscopic surgical procedures. Laparoscopic surgery offers the advantage of less tissue disruption, quicker recovery times, and shorter periods of postoperative immobilization. Intuitively the reduction in tissue damage and the possibility of faster mobilization predicts lower risk of thromboembolic complications. Conversely, patients undergoing laparoscopic procedures are subjected to increased venous stasis as a result of the induction of pneumoperitoneum and prolonged use of the reverse Trendelenburg position to visualize and manipulate internal organs.6 The American College of Chest Physicians’ (ACCP) Clinical Practice Guidelines (8th edition) recommend against routine prophylaxis (other than early and frequent ambulation) in patients undergoing laparoscopic surgery without thromboembolic risk factors, and recommends mechanical or pharmacologic prophylaxis in patients with any thromboembolic risk factors.7 The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) guidelines for DVT prophylaxis during laparoscopic surgery stratifies inpatients into low, moderate, and high risk groups for thrombosis on the basis of a risk score imputed from the type of procedure and patient risk factors.8,9 Procedure-related risk factors include procedure lasting over 1 hour, and pelvic procedures. Patient-related factors include age > 40 years, immobility, malignancy, thrombophilic states (protein C, protein S, or ATIII deficiency), obesity, peripartum state (or use of estrogens), heart failure, renal failure, varicose veins, inflammatory states, or infection. In the lowest risk group (procedure < 60 minutes in patients with no risk factors) elastic stockings and early ambulation is all that is recommended, and UFH or LMWH is optional. In the moderate-risk group (one patient risk factor in a procedure of less than 60 minutes, or any procedure > 60 minutes with no patient risk factors) pneumatic compression devices or prophylactic heparin or LMWH are recommended. In the high-risk group (two or more risk factors in procedures >60 minutes) a combination of serial compression devices and prophylactic UFH or LMWH are recommended.8,9
Once-daily LMWH appears to be as safe and effective as multiple daily injections of UFH and provides convenience as well as a better quality of life for the patient.3,4 In the Clinical Center at the National Institutes of Health, enoxaparin is the LMWH most commonly employed. However, fondaparinux or other LMWHs, such as nadroparin, dalteparin, ardeparin, tinzaparin, and reviparin, may be considered equivalent. The administration of warfarin at a low, fixed dose (e.g., 1 mg/day) has not been shown to be of value for VTE prophylaxis, and is not recommended.
Primary Venous Thromboembolism Prophylaxis in Cancer Patients Receiving Chemotherapy, Hormonal, and/or Antiangiogenic Treatment
Patients with cancer who are undergoing treatment should be considered for VTE prophylaxis if they have one or more of the following: a history of VTE, a large mass compressing a major vessel, or treatment which includes tamoxifen/raloxifene, diethylstilbestrol, or chemotherapy, especially use of bevacizumab, thalidomide- or lenalidomide-based combination regimens, particularly those given in combination with high-dose dexamethasone.10 A recent clinical trial of patients with advanced cancer of lung, gastrointestinal, pancreatic, breast, ovarian, or head and neck undergoing chemotherapy (PROTECHT trial) showed a statistically significant (P = 0.02) decrease in thromboembolic events from 3.9% to 2.0% in the groups receiving prophylactic LMWH (such as nadroparin) or placebo, respectively.11 VTE prophylaxis in cancer patients undergoing treatment should be individualized; if prophylaxis is chosen, LMWH (enoxaparin 40 mg/day) or UFH (low dose) should be considered (Table 23.3). Aspirin prophylaxis (81–325 mg daily) is an option for patients receiving thalidomide or lenalidomide for multiple myeloma.10 In chronic lymphocytic leukemia (CLL) patients treated with lenalidomide it was shown that TNFα, C-reactive protein, factor VIII, thrombomodulin, and sVCAM1 were significantly increased from baseline after initiation of treatment (P < 0.001), and TNFα and sVCAM levels were more elevated in patients who subsequently had DVTs, suggesting inflammation and endothelial cell dysfunction played an important role in VTE risk.12 Thus, anti-inflammatory effects of aspirin may contribute to prophylaxis against VTE in addition to antiplatelet effects otherwise not thought to be important for VTE prophylaxis.
Primary Prophylaxis in Immobilized/Hospitalized Cancer Patients
VTE is a fairly common event in hospitalized cancer patients. A retrospective study of over 66,000 hospitalized neutropenic adult cancer patients showed that 3% to 12% of these patients, depending on the type of malignancy, experienced VTE during their first hospitalization.13 Primary prophylaxis is effective in hospitalized medical patients, who undergo a three-fold reduction in VTE when treated with enoxaparin at a daily dose of 40 mg, compared to control patients receiving no treatment. This conclusion is derived from the MEDENOX trial,14 a double-blind randomized study of 1,102 patients with acute medical illnesses who received prophylaxis against VTE (14.9% of these patients had cancer or a history of cancer). Patients were randomized to one of three groups that would receive for 6 to 14 days subcutaneous daily administration of 40 mg of enoxaparin, 20 mg of enoxaparin, or a placebo. The primary outcome was VTE during the ensuing 3 months. The data favored prophylactic treatment with subcutaneous enoxaparin at a dose of 40 mg daily. Adverse events, which included hemorrhage, local reaction, thrombocytopenia, and death from any cause, were not different between the groups receiving enoxaparin and placebo. The weakness of this study was that a more appropriate control group would have received UFH. Therefore, it has been recommended that all hospitalized patients with cancer should receive anticoagulation therapy in the absence of contraindications. A subsequent randomized trial, the LIFENOX Trial, indicates, however, that all-cause mortality is unchanged in medical patients undergoing thromboprophylaxis with LMWH.15 UFH is the agent of choice for thromboprophylaxis in hospitalized patients with a creatinine clearance of < 30 mL/min. A reduced enoxaparin dose of 30 mg daily can also be used in this situation and is preferred if prolonged use is required. Occasional monitoring of anti-factor Xa levels may be appropriate in the setting of renal failure to prevent overdosage and bleeding.
Primary Prophylaxis in Patients with Brain Metastases and Primary Brain Tumors
The risk of VTE in patients with primary or metastatic brain tumors is increased for various reasons including expression of tissue factor16 and PAI-117 by gliomas, immobility due to paresis of limbs affected by the brain tumor or metastasis. Additionally, the use of antiangiogenic agents such as Avastin™ (bevacizumab) may further increase the risk of arterial thrombosis and ironically increase the risk of bleeding.18,19 The challenge in using anticoagulation is balancing the risk of thrombosis with the risk of precipitating intracranial hemorrhage. Studies have shown both increased risk as well as benefit with the use of LMWH prophylaxis in the nonsurgical setting.20
For patients undergoing neurosurgery, the recommended prophylaxis is to initiate LMWH or low-dose UFH 24 hours postoperatively, in combination with mechanical thromboprophylaxis, such as graduated compression stocking and/or intermittent pneumatic compression. This is associated with minimal risk for bleeding.21 Initiation of prophylaxis before neurosurgery in patients with brain tumors may be associated with increased risk for intracranial hemorrhage, as shown in one such study terminated early due to increased bleeding.22
Treatment of Venous Thromboembolism in Patients with Primary Brain Tumors or Brain Metastases
Patients with primary brain tumors or metastases who develop VTE can be treated will full doses of UFH or LMWH.23 Here the use of UFH has the potential advantage over LMWH of a short half-life and the ability to administer protamine to neutralize it in the event of hemorrhage or overdosage. It may be advisable to forego the use of a bolus at the outset of treatment and simply start an infusion and increase the rate with frequent monitoring to prevent inadvertent overdosage. We prefer the use of anti-factor Xa activity monitoring (rather than the aPTT) due to the possibility that the aPTT is not as accurate in its ability to predict anticoagulant effects of heparin. Lupus anticoagulants or increased factor VIII levels may result in spuriously long or short values, respectively. A screening noncontrast head computed tomography (CT) may be considered to exclude recent intracranial bleeding before the initiation of anticoagulation,23 especially in patients with certain types of brain metastases associated with high rates of spontaneous hemorrhage, such as with thyroid cancer, melanoma, renal cell carcinoma, and choriocarcinoma. Evidence of recent spontaneous bleeding is generally considered a contraindication to anticoagulation. IVC filters have a role in this situation, but their placement is often proposed on the mistaken assumption that the patient cannot be anticoagulated, and may be overused.
Treatment of Patients with Trousseau Syndrome
Trousseau syndrome is the constellation of venous and arterial thromboembolic disorders predating or associated with a malignancy.24,25 Patients with this syndrome, even if anticoagulated with warfarin with a therapeutic international normalized ratio (INR), may nevertheless have recurrent thrombi. Other clinical characteristics of Trousseau syndrome include microangiopathy, chronic, low-grade disseminated intravascular coagulation (DIC), and nonbacterial thrombotic endocarditis. UFH, LMWH, and fondaparinux are more effective than warfarin in the treatment of Trousseau syndrome. The dosing of anticoagulation varies depending on the clinical setting. For example, a patient with an acute DVT will require enoxaparin at therapeutic doses, whereas DIC may be controlled with lower doses. Treatment is administered indefinitely (or for as long as tumor persists).
General Approach in Treating Venous Thromboembolism in Cancer Patients
In general, treatment of VTE in patients with cancer consists of acute therapy with LMWH or UFH for at least 5 to 7 days’ duration in patients without contraindications to anticoagulation followed by LMWH or warfarin for at least 3 months. The CLOT trial26 showed an 8% absolute risk reduction without an increase in major bleeding when cancer-related VTE was treated with an LMWH, for example, dalteparin for 6 months compared with warfarin. Chronic therapy with LMWH is associated with superior outcomes in cancer patients with VTE.
Cancer patients with VTE should be treated for a minimum of 3 months, while patients with PE should be treated for at least 6 months, ideally with LMWH. Anticoagulation for an indefinite duration may be considered in patients with active cancer or persistent risk factors who may be bedridden, critically ill, and/or malnourished. Extended anticoagulation therapy with an LMWH may require dosage reduction after an initial period. For example, in the CLOT study, the dalteparin dosing was lowered from 200 units/kg every day to 150 units/kg every day after 1 month.
In the event that warfarin will be used for chronic therapy (due to cost or patient’s preference), there should be a transition phase of at least 5 days during which the acute parenteral anticoagulant (e.g., UFH, LMWH, or fondaparinux) is overlapped with warfarin until an INR of 2.0 or more is achieved. Clinicians should be aware that the warfarin modulation of anticoagulation intensity can be clinically challenging due to drug-drug interactions with commonly used chemotherapeutics, antimicrobials, and other new drugs such as those undergoing testing in Phase I clinical trials.
Most institutions have nomograms for dosing and monitoring of UFH. Anti-factor Xa activity instead of the aPTT has been more frequently used to monitor UFH because of the observation of dissociation between the aPTT and heparin levels measured by anti-factor Xa activity, suggesting heparin resistance. Heparin resistance usually occurs in patients with elevations in factor VIII or von Willebrand factor, antithrombin III (AT) deficiency, increased heparin clearance, elevations in heparin-binding proteins, and use of fibrinogen. Factor VIII, von Willebrand factor, and fibrinogen are acute-phase proteins and elevated factor VIII levels shorten the aPTT.27–29 When UFH is used, the target therapeutic anti-factor Xa level should be 0.3 to 0.7 U/mL.
Anticoagulation Options in Cancer Patients
In patients who develop recurrent VTE despite adequate anticoagulation with warfarin (INR 2.0–3.0) the etiology may be cancer-related hypercoagulability such as the Trousseau syndrome, anatomic causes such as extrinsic vascular compression, and acquired or familial thrombophilia. Treatment can be changed to heparin (LMWH preferred) or fondaparinux. The use of heparin is preferred to use of vitamin K antagonists in the setting of cancer.30 Switching to heparin therapy is an option following failure of fondaparinux to prevent VTE recurrence and vice versa. Twice-daily dosing of enoxaparin is an option for patients exhibiting recurrent VTE while receiving once-daily therapy with am LMWH,31 and escalating the dose of LMWH can be effective for treating cases that are resistant to standard, weight-adjusted doses of LMWH32 If thrombocytopenia occurs during anticoagulation, chemotherapy-induced thrombocytopenia, DIC, heparin-induced thrombocytopenia (HIT), antiphospholipid antibody syndrome (APS), thrombotic thrombocytopenic purpura, immune thrombocytopenic purpura, bone marrow failure, and folate or vitamin B12 deficiency should all be part of the differential diagnosis. Thrombocytopenia does not protect against thrombosis. Anticoagulation therapy should not be withheld because of relative thrombocytopenia alone. The management of antithrombotic therapy in patients with thrombocytopenia requires individualized assessments of the risk of bleeding and the risk of thrombosis.33 Low-dose enoxaparin (< 1 mg/kg/day) may be considered safe at a platelet count in the range of 20 and 55 × 109/L in stem cell transplantation patients who weigh >55 kg.34 On the other hand, thrombocytopenia in APS and HIT may indicate increased disease activity and increased thrombotic potential and aggressive antithrombotic therapy may be warranted.35 Clinical suspicion of HIT should be high when recurrent VTE is observed in a cancer patient receiving or recently exposed to heparinbased therapy. In the typical presentation, platelet counts fall by more than 50% from baseline 5 to 8 days after exposure to heparin. The drop in platelet count can occur even sooner if the patient has been primed by treatment with heparin prior to the current exposure. A major difficulty in diagnosis of HIT is that cancer patients often have multiple reasons for thrombocytopenia, including myelosuppressive drugs, radiation therapy, and infections. An algorithm for calculating pretest probability of HIT includes clinical elements such as presence of thrombocytopenia, timing of drop in platelet count, other causes for thrombocytopenia, and the presence of thrombosis36 and has been modified since its introduction to improve the accuracy of pretest probability estimation.37 Testing for antiplatelet factor 4 antibodies with a sensitive ELISA method can exclude HIT if negative. In cases where the result is positive, the possibility of a false positive can be ruled out by the more specific test for release of 14
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree