Semen parameter (according to [1])
Testicular cancer patients (n = 563) [2]
Patients with Hodgkin lymphoma (n = 474) [3]
Normozoospermia
25 %
41 %
Oligozoospermia
56 %
56 %
Azoospermia
14 %
3 %
Anejaculation
5 %
n.a.
Patients with bilateral TGCC or unilateral cancer with contralateral TIN will have worse results in semen analysis, and the rate of azoospermia may drastically rise [4, 5].
Finally, impaired spermatogenesis results in reduced paternity rates in testicular cancer patients. Prior to any further treatment, about 40 % of men are proven fathers at the time of diagnosis. Of course, due to young age at diagnosis, testicular cancer patients very often do not have yet finalised family planning and are therefore childless. However, paternity rates in long-term survivors of testicular cancer remain also reduced (about 30 % lower) in comparison to the normal male population. A median time interval of about 7 years goes by until pregnancies are induced. But even 12 years after end of treatment, recovery of fertility may be observed. In 3,005 long-term survivors of TGCC, 1,460 (49 %) achieved paternity after a median follow-up between 5 and 12 years [6] (Table 12.2). Negative prognostic parameters are the application of radiotherapy and a treatment period before 1990. FSH may help to indicate recovery of spermatogenesis, but is not reliable. Pregnancies are more often reported in patients with normal FSH serum levels and antegrade ejaculation compared to those with elevated FSH. Inhibin B does not improve prediction of fertility and is thus only recommended in clinical studies and not for routine analysis. However, in prediction of persistent posttreatment azoospermia, it may be of some value [15]. For testicular cancer patients no increased risk for malformations in offspring is reported [11].
Table 12.2
Paternity rates in testicular cancer survivors
Patient numbers | Median FU (years) | Paternity (spontaneous) (%) | Paternity (with ART) (%) | Reference |
|---|---|---|---|---|
246 | 73 (29.7 %) | k.A. | [7] | |
88 | 7 | 52 (59.0 %) | 7 (13.5 %) | [8] |
164 | 8 | 110 (67.1 %) | n.a. | [9] |
207 | ≥5 | 159 (81.7 %) | 10 (6 %) | [10] |
552 | 11 | 325 (65.5 %) | 36 (10 %) | [11] |
1,359 | 5 | 513 (37.6 %) | 14 (1.0 %) | [12] |
143 | 12 | 85 (59.4 %) | n.a. | [13] |
246 | 83 (33.7 %) | n.a. | [14] | |
3,005 | 1,393 (46.4) | 67 (2.6 %) | Total numbers published until 2010 |
Recovery of spermatogenesis and thus fertility cannot be safely predicted for individual patients prior to treatment. Brydoy et al. [13] published data on 100 % paternity in men after two cycles PEB treatment – however, even severely reduced spermatogenesis may be observed after one cycle of carboplatin treatment. Thus the individual susceptibility to the gonadotoxic agents cannot be foreseen and make counselling of patients difficult. Most data on fertility issues are retrospectively obtained. Recently, a cohort of 213 patients with TGCC with a mean follow-up of 48 months was analysed. They had cryopreserved their semen prior to treatment [17]. Altogether, 18.8 % of these men revealed persistent azoospermia during follow-up; another 4 % had cryptozoospermia with sperm counts <0.1 mill/ml and thus no chance for spontaneous pregnancy induction. Seventy-seven percent of men recovered with either oligozoospermia or normozoospermia. There was no difference in patients with seminomas or non-seminomas. Radiotherapy was associated with higher rates of azoospermia (34 % compared to 12.5 % without radiotherapy). Moreover, a clear association could be determined between initial semen quality and potential for recovery: the lower the sperm concentration prior to treatment, the higher the rate of posttreatment azoospermia [17].
12.2 Fertility Preservation in Testicular Cancer Patients
Due to the known negative long-term effects of TGCC and its treatment on fertility aspects, counselling of patients in respect to fertility preservation is mandatory. Apart from the medical indication, there exists a legal indication to inform the patients at the time of diagnosis of the different aspects of fertility impairment and to offer fertility preservation. The standard procedure comprises the cryopreservation of semen [5]. Semen samples can be easily cryopreserved in small straws with acceptable survival rates of spermatozoa if handled according to standardised protocols as described in detail in the WHO laboratory manual [18]. The cryopreserved spermatozoa can later be used for assisted reproduction, namely, intracytoplasmic sperm injection technique (ICSI). If patients are adequately informed, about 50 % of men will cryopreserve spermatozoa [12]. About 20 % of men will not be able to cryopreserve semen with spermatozoa, either due to anejaculation or azoospermia. In these cases, alternatively a surgical testicular sperm extraction (TESE) can be offered [5]. The best method nowadays is the microsurgical approach, but also a multifocal approach is still an accepted standard in case of non-obstructive azoospermia [19]. TESE can be successfully performed if spermatogenic foci within the testicular parenchyma can be isolated. The testicular spermatozoa can be cryopreserved for later use in assisted reproduction (ICSI). TESE procedures in testicular cancer patients with azoospermia can be successful in 61 % of patients [2]. Especially patients with bilateral tumour manifestations (TGCC or TIN) will benefit from additional TESE options prior to (functional) anorchia.
In general, semen cryopreservation should be offered prior to primary treatment including inguinal orchiectomy. Results of semen analysis will be better in quality and quantity before surgery is performed. Moreover, semen analysis prior to orchiectomy will easily detect patients with azoospermia that will need additional TESE for fertility preservation. Thus, treatment schedule can be optimised, and primary surgery can immediately be combined with testicular sperm extraction. In summary, patient counselling in respect to fertility issues as well as cryopreservation of spermatozoa improves quality of life and should be an accepted standard [5].
12.3 Hypogonadism
Testosterone is the central male hormone with numerous positive effects on male physical and mental well-being. Testosterone deficiency may result in pathological effects. Values above the accepted lower normal range of 12.1 nmol/l total testosterone can be expected in healthy men. Testosterone levels <12 nmol/l and the existence of clinical symptoms of testosterone deficiency result in serious changes in body composition, reduced muscle strength, increase in fat mass and osteopenia [19]. Very often hypogonadism is accompanied by insulin resistance and disturbed lipid metabolism [20]. There exist several studies that revealed hypogonadism to be a relevant late effect of TGCC treatment. Depending on treatment intensity, the incidence varies between 11 and 33 % ([21], Table 12.2). In up to 34 % of men, a compensated Leydig cell insufficiency with high LH and low normal testosterone levels could be demonstrated in recent studies [10, 22]. There exist no relevant differences between seminomatous and non-seminomatous tumours (Fig. 12.1) [21]. Irrespective of treatment modalities hypogonadism may develop (Table 12.3) [21], although an association between decrease of testosterone levels and intensity of treatment was shown [22]. A recent prospective follow-up study revealed an incidence of 40 % in patients with manifest or testosterone-treated hypogonadism [23].
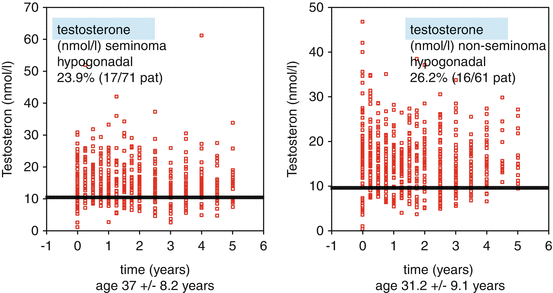

Fig. 12.1
Testosterone deficiency in testicular cancer patients 4.8 years after therapy (Modified according to Pühse et al. [21])
Several potential risk factors predictive for hypogonadism could be evaluated: reduced testicular volume (<12 ml), microlithiasis of the testis, >2 cycles of polychemotherapy and pre-existing low testosterone serum levels [21, 24]. However, these findings are inconsistent and may vary between different study populations.
In case of hypogonadism defined by low testosterone serum levels and clinical symptoms, testosterone substitution is indicated if contraindications are excluded. Testosterone treatment is accepted as safe and can be effectively performed by either transdermal or intramuscular application according to the current guidelines [25].
In case of bilateral TGCC and anorchia, testosterone substitution has to be initiated immediately after surgery. In case of unilateral TGCC and contralateral TIN or bilateral TGCC with organ-sparing surgery, testosterone substitution can be delayed if serum levels still remain in the normal range without symptoms of androgen deficiency.
12.4 Metabolic Syndrome (MBS)
The components of the metabolic syndrome can significantly more often be observed in patients after TGCC than in controls: overweight in 24–50 %, hypercholesterinemia in 24 % and high blood pressure in 30 % of men are present after 1–5 years after diagnosis of TGCC. The MBS can be observed in about 25 % of men with TGCC and increases up to 35 % at the age of 40–60 years [26, 23].
Recently, the close interaction between hypogonadism and metabolic syndrome was shown. In case of testicular cancer patients, the young age may result in worse results and possibly add to the increased cardiovascular risk factors documented as late effects in long-term survivors and thus increase morbidity [26–28] [23]. From endocrinological studies we learned that the treatment of hypogonadism may result in normalised mortality rates comparable with eugonadal men, while untreated hypogonadal men die earlier [29]. To what extent the early detection and treatment of hypogonadism will positively influence the development of the metabolic syndrome and its late effects, especially on the cardiovascular system, cannot be judged by now. Data from different patient populations suggest synergistic positive effects by testosterone substitution on metabolism and thus morbidity and mortality rates [29].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree