Drug
Dose
Route of administration
Side effects
Diazoxide
5–20 mg/kg/day divided into three doses
Oral
Fluid retention, hypertrichosis
Chlorothiazide (in conjunction with diazoxide)
7–10 mg/kg/day divided into two doses
Oral
Hyponatraemia, hypokalaemia
Glucagon
1–10 μg/kg/h 0.5–1 mg for emergency treatment of hypoglycaemia
SC/IV infusion IM/IV
Nausea, vomiting, skin rashes.
Paradoxical hypoglycaemia in high doses
Octreotide
5–35 μg/kg/day
SC as a continuous infusion or 6–8 hourly injections
Common—tachyphylaxis
Others—Suppression of GH, TSH, ACTH, glucagon; diarrhoea, steatorrhoea, cholelithiasis, abdominal distension, growth suppression
Histological Subtypes of Phhi
Histologically, there are two major subtypes of CHI, diffuse and focal [4] (Fig. 12.1). However, patients have been described with atypical histological forms of CHI which do not fit into the classification of diffuse or focal [16, 17]. The diffuse form of CHI affects all the ß-cells within the islets of Langerhans (Fig. 12.2). The histological subtype of CHI can be a guide as to the mode of inheritance. Diffuse disease can be familial or sporadic and can result from recessively inherited or dominantly acting mutations in the genes previously described whilst focal disease is always sporadic [18]. The diffuse and focal forms of CHI are histologically unrelated to nesidioblastosis [19] (which describes the proliferation of islets cells budding off from pancreatic ducts).
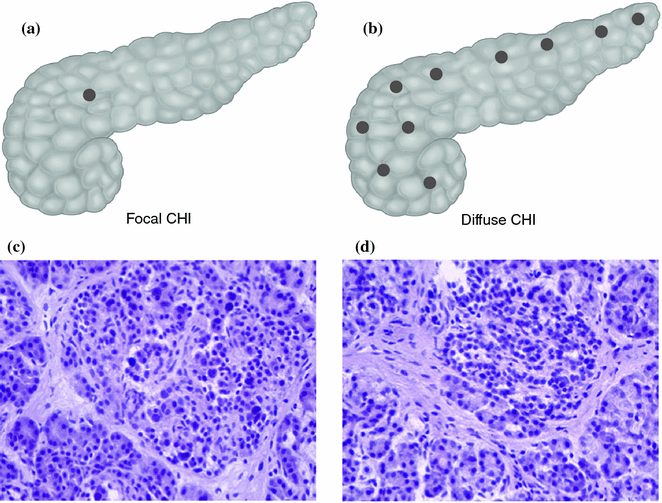
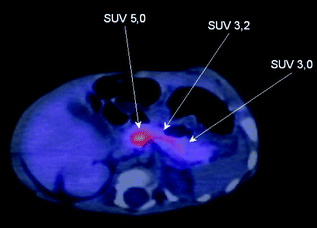

Fig. 12.1
Histological subtypes of CHI. a and b The focal form is localised to a single region of the pancreas whereas the diffuse form affects the whole pancreas. c and d On H&E staining there is marked hyperplasia of the islets in focal disease

Fig. 12.2
18F-DOPA-PET/CT scan of focal lesion located in the head of the pancreas. The standard uptake value (SUV) of Fluorine-18-l-3,4-dihydroxyphenylalanine is highest in the head of the pancreas (5.0) compared to the body and tail of the pancreas. At surgery a focal lesion was found located between the superior mesenteric vein and the portal vein
Focal pancreatic lesions appear as small regions of islet adenomatosis (nodular hyperplasia of islet-like cell clusters, including ductuloinsular complexes) measuring 2–10 mm which are characterised by ß-cells with enlarged nuclei surrounded by normal tissue [18, 20] (Fig. 12.2). Focal disease results from paternal uniparental disomy (UPD) encompassing chromosome11p15.5-11p15.1 within a single pancreatic ß-cell which unmasks a paternally inherited KATP channel mutation at 11p15.1 [21, 22]. Paternal UPD at 11p15.5 causes altered expression of a number of imprinted genes, including the maternally expressed tumour suppressor genes H19 and CDKN1C, and the paternally expressed growth factor IGF2, likely leading to clonal expansion of the single cell and dysregulated insulin secretion from the resulting focal lesion [21, 22].
Differential of Focal and Diffuse
In patients that are unresponsive to medical therapy with diazoxide it is essential to differentiate focal from diffuse disease as the surgical approaches are radically different [23]. Infants with diffuse and focal disease are clinically and biochemically undistinguishable, thus there is no biochemical marker which will help in the differentiation. The precise preoperative localisation and limited surgical removal of the focal domain “cures” the patient from the hypoglycaemia and minimizes the risk of pancreatic exocrine insufficiency. Focal lesions are typically invisible to the naked eye at the time of the operation (size can vary from 2 to 10 mm in diameter) and in most cases buried deep within the pancreas [4]. In contrast, patients with diffuse disease will require a near total pancreatectomy which will have life-long implications (with a high risk of developing diabetes mellitus and pancreatic exocrine insufficiency). Patients with diffuse CHI that are medically managed also have a high risk of developing diabetes mellitus [16].
In the past methods for identifying children with a focal CHI included intrahepatic pancreatic portal venous sampling (PVS), arterial calcium stimulation/venous sampling, acute insulin response testing to intravenous glucose, calcium and tolbutamide and biopsy of the tail of the pancreas. However, these investigations are not only technically difficult and challenging but also have poor sensitivity and specificity. Focal lesions cannot be visualized with magnetic resonance imaging (MRI) or computerized tomography (CT) scans.
However, recent advances in 18F-DOPA-PET (Fluorine-18-l-3,4-dihydroxyphenylalanine positron emission tomography) imaging have completely changed the diagnostic approach to these patients [24–30]. The principle of this radiological technique is based on the fact that pancreatic islets take up l–DOPA and convert it into dopamine by the aromatic amino acid decarboxylase [28]. The uptake of the positron emitting tracer 18F-DOPA is increased in ß-cells with a high rate of insulin synthesis and secretion (increased metabolism) compared to unaffected areas. Dopamine receptors are expressed in pancreatic ß-cells suggesting a possible role in regulating insulin secretion. However, the precise role of dopamine in the pancreatic ß-cells is still unclear.
The detection limit of PET imaging using 18F-DOPA-PET is equivalent to 105–106 cells and a diameter of approximately 1 mm [6]. The recent introduction of the integrated 18F-DOPA-PET/CT technique allows more precise preoperative localization of the focal lesion [31]. The new 18F-DOPA-PET/CT technique allows visualization of the splenic artery, portal vein, superior mesenteric and inferior mesenteric arteries as well as the duodenum and enables exact anatomical and functional description of the pancreatic focus [31]. Ectopic focal lesions (in the wall of the jejunum and the ileum) can also be detected by 18F-DOPA-PET [32, 33]. 18F-DOPA-PET/CT scan is indicated in those patients who have the genotype of a focal lesion (namely a paternally inherited mutation in ABCC8 or KCNJ11) or have no mutations in the ABCC8/KCNJ11 genes. If the patient has a homozygous or compound heterozygote mutation in ABCC8 or KCNJ11 then this is likely to be associated with diffuse disease and an 18F-DOPA-PET/CT is not required these cases. The aim of the 18F-DOPA-PET/CT is to allow pre-operative localisation of the focal domain [25, 26]. We recently reviewed our experience in the PET-CT localization of focal CHI and this suggests that in focal lesions, 18F-DOPA-PET scan is useful in defining the site and dimension of the focal lesion in 2/3 of patients [34].
Surgery for CHI
Surgery is indicated for cases of diffuse CHI which are medically unresponsive (diazoxide and octreotide) and in all cases of focal disease provided that the focal lesion is accurately localised pre-operatively. A multidisciplinary team (involving the endocrinologist, histopathologist and surgeon) approach to patients with CHI can help in distinguishing focal from diffuse disease, locate focal lesions, and permit partial pancreatectomy with cure in most patients [5, 35]. It is recommended that surgery for CHI patients should be done in centers of expertise where all the facilities and resources are available. The surgeon has to work very closely with the histopathologist as intra-operative examination of pancreatic specimens will guide the surgeon on the extent of resection required [36]. Surgeons need to be aware about the limitations of 18F-DOPA-PET in identifying the exact location and the dimension of the focal lesion and should always rely on the intra-operative histological confirmation of complete excision of the focal lesion [34].
Surgery for Diffuse CHI
The extent of pancreatic resection for diffuse disease is controversial, however most of the current literature recommends a near total or 95% pancreatectomy [23]. The aim of doing a near total pancreatectomy for diffuse disease ideally is to remove enough pancreatic tissue so that the child can become independent of intravenous glucose infusion and maintain normoglycaemia on bolus enteral feeds. However, there is a very delicate balance between removing too much or too little pancreatic tissue. The child will still have hyperinsulinaemic hypoglycaemia following post pancreatectomy if too little pancreatic tissue is removed (which may require another pancreatectomy) and early diabetes mellitus will develop if too much tissue is resected. There are currently no markers which can be used during the intra-operative period to direct the extent of pancreatic resection. Thus not having a precise marker for the extent of pancreatic resection makes surgery complicated for diffuse disease. In near-total (95%) pancreatectomy, the tail, body, uncinate process and part of pancreatic head are resected, leaving pancreatic tissue surrounding the common bile duct and along the duodenum [37] (Fig. 12.3).
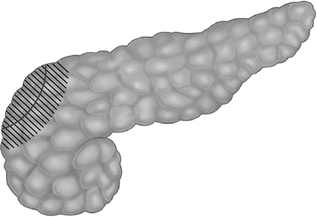

Fig. 12.3
Near-total pancreatectomy. The shaded area indicates the remaining pancreas after resection, leaving behind pancreatic tissue around the common bile duct and along the medial border of the duodenum
Open Near-Total Pancreatectomy
Pancreatic resection for the diffuse type of CHI has traditionally been carried out by the open approach. The abdomen is accessed via a generous transverse supraumbilical incision. The lesser sac is entered through the gastrocolic omentum; Kocherisation of the duodenum allows full exposure of the pancreas. The tail of the pancreas is sent for intraoperative frozen section histology and the diagnosis of diffuse CHI is confirmed before proceeding with pancreatectomy.
A stay suture is placed in the tail of the pancreas to allow traction of the pancreas facilitating its dissection since direct handling of the pancreas results in fracture of this friable organ. The tail of the pancreas is carefully dissected away from the hilum of the spleen, and the short pancreatic vessels are coagulated. Dissection of the pancreas is carried out in a medial direction towards the head of the pancreas. Pancreatic vessels passing from the splenic vessels into the pancreas are coagulated and divided using bipolar diathermy. Meticulous care is taken with the splenic artery and vein which are closely related to the pancreas, as the spleen should be preserved. Once dissection has arrived at the superior mesenteric vessels, the uncinate process is mobilised. A sling may be placed around the superior mesenteric vessels, retracting them away from the uncinate process.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree