© Springer International Publishing AG 2018
Laurence Zitvogel and Guido Kroemer (eds.)Oncoimmunologyhttps://doi.org/10.1007/978-3-319-62431-0_4343. Concluding Remarks
(1)
Ludwig Cancer Research Center at University of Lausanne, Switzerland and Cordeliers Research Center, Paris, France
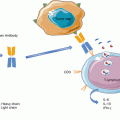
In the 1890s, a surgeon working at what today is known as the Memorial Sloan Kettering Cancer Center in New York had the momentous insight that the regression of a relapsing sarcoma observed in a patient undergoing erysipelas, as a complication of surgery, could have been a case of tumor rejection triggered by the body defenses that had been activated against the infection. W. Coley, as was his name, decided to test the validity of this idea by inoculating laboratory-grown bacteria, the state of the art in microbiology in those days, directly into the tumors and, to his amazement, observed complete tumor responses in some of the treated patients. These results ignited a new field of research known as tumor immunology. It took 120 years for this branch of immunology to, together with cancer biology and clinical oncology, fully come of age. The scientific and oncology communities have driven a renaissance of the field in the last 10 years. The major successes of immune checkpoint blockade during the last 5 years have reignited the field in a major way. To date, immunotherapy is becoming the fourth pillar of cancer treatment joining surgery and chemo- and radiotherapy. Monoclonal antibodies blocking the PD-1/PD-L1 axis have emerged as the backbone of immunotherapy in various tumor types including melanoma, lung, renal, bladder, and Hodgkin’s lymphoma.
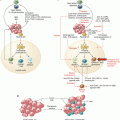
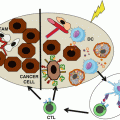
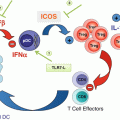
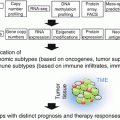
What have we learned? We now know that the immune system often recognizes tumors early during carcinogenesis up, in some cases, to the metastatic state. Strong supports for this statement are the favorable prognostic impact of Th1-oriented and cytotoxic T cell infiltration and the identification of T cells specific for mutated tumor antigens in the tumor microenvironment. The interactions between evolving and developing tumors and the immune system are dynamic and lead to a reciprocal and progressive sculpting. Tumor variants selected upon immune pressure evolve resistance to the main mechanisms of antitumor immunity, such as IFNγ or cytolytic lymphocytes. Conversely, various components of innate and adaptive immunity may be co-opted by tumors to provide niches favorable to their growth, migration, invasion, and seeding at distant sites. In clinically manifest tumors, a significant fraction of them are “T cell inflamed.” The proportion of tumors that are infiltrated by T cells is variable from one tumor type to the other and within patients bearing cancers with similar histology. Many reasons may explain this variability. The intrinsic immunogenicity of tumors may play a significant role including tumors with a high load of somatic mutations, such as melanoma or lung cancer, which are likely to display a high density of neoantigens to T cells. However, an intact dendritic cell compartment, in particular cross-presenting BATF3+ dendritic cells, is required for neoantigens to be “visible” to the host’s immune system. Moreover, in addition to the intrinsic tumor immunogenicity and the antigen processing and presentation arm, the access to tumors by migrating T cells is critical. The endothelial cells lining the neovessels are a barrier which primed T cells need to cross in order to infiltrate tumors. They go across the endothelial cell walls by a well-regulated set of mechano-cell biological processes involving sequential rolling along the vessel wall, tethering, stopping, and crawling across the endothelial cell junctions until reaching the tissue space beyond tumor blood vessels. Extravasated T cells then need to migrate into the tumor parenchyma. In short, variable and relatively high proportions of cancers are so-called immune deserts owing to the many steps in the immune cell infiltration process that may be disrupted in the advanced tumors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree