(1)
RadioOnkologie und Strahlentherapie, UniversitätsKlinikum Heidelberg, Im Neuenheimer Feld 400, Heidelberg, 69120, Germany
9.1 Curative Treatment Concepts
9.1.1 Axillary Cancer of Unknown Primary
9.1.1.1 Introduction
Axillary cancer of unknown primary (CUP) is defined as the infiltration of axillary lymph nodes by (adeno) carcinoma without the detection of a primary tumor on standard imaging. The first three cases of axillary CUP were published by William S. Halsted. The incidence of axillary CUP ranges between 0.12 and 0.67 % of all malignant neoplasms [2]. Due to this fact, practically all publications are based on retrospective case series, mostly from single institutions, and many treatment approaches have been deduced from the treatment of breast cancer patients.
Diagnostic imaging plays a key role in the establishment of a diagnosis of CUP. In a meta-analysis by Pentheroudakis et al., the lack of sensitivity of historic imaging modalities is reflected by the fact that in patients with suspected axillary CUP who had mastectomy, a clinically occult primary tumor could be identified in 72 % of the specimens [2]. Recently, the introduction of MRI has changed the diagnostic management of axillary CUP substantially. Pooled sensitivity and specificity were 90 and 31 % in a systematic review of breast MRI [3].
9.1.1.2 General Management
Although some older studies have used axillary lymph node sampling followed by axillary radiotherapy, axillary lymph node dissection (ALND) has been considered a standard procedure due to an improved locoregional control rate and the prognostic relevance of accurate axillary staging [2].
The optimal treatment of the ipsilateral breast is controversial. Available options are observation, mastectomy, and whole breast radiotherapy. In a survey of the American Society of Breast Surgeons, 43 % voted for mastectomy, 37 % preferred whole breast radiotherapy, and 6 % recommended observation [4].
Observation after axillary surgery is associated with unacceptably high rates of locoregional relapse. In a meta-analysis by Pentheroudakis et al., 42 % of patients developed a recurrence in the ipsilateral breast after a median latency period of 4–64 months. Other retrospective series have shown locoregional recurrence rates of up to 70–80 % after observation only [5–9].
Local treatment of the breast by mastectomy can improve locoregional control and also disease-free and overall survival. In a retrospective study on 51 patients, Wang et al. could show that mastectomy compared to observation reduced the risk of any recurrence from 77 to 26 % while improving median overall survival from 23 to 76 months [10]. Similarly, Blanchard and Farley showed significantly better disease-free and overall survival in patients undergoing ipsilateral mastectomy compared to observation [11].
9.1.1.3 Breast-Conserving Treatment
In the 1980s, first reports of breast-conserving treatment in axillary CUP were published [12]. In analogy to breast-conserving therapy in breast cancer, where adjuvant radiotherapy reduces the risk of any recurrence by approximately 50 % leading to an absolute survival benefit of 4 % after 15 years [13], whole breast radiotherapy was added after axillary dissection to eradicate clinically occult disease in the ipsilateral breast. In the first reports, 10-year survival rates of 72 % were achieved while breast preservation was feasible in 75 % of patients [12]. Since then, numerous studies on breast-conserving treatment in patients with axillary CUP have been published (for a summary, see Table 9.1).
Table 9.1
Studies comparing observation, radiotherapy, and mastectomy after axillary lymph node dissection for patients with axillary CUP
Author | Study period | N | Follow-up | LRC (Obs/RT/M) | OS (Obs/RT/M) | ||||
|---|---|---|---|---|---|---|---|---|---|
Barton [5] | 1975–2009 | 48 | 68 m | 34 % | 87 % | – | 83 % | 92 % | – |
Ellerbroek [7] | 1944–1987 | 42 | 131 m | 43 % | 83 % | – | – | – | – |
Foroudi [6] | 1979–1996 | 20 | 73 m | 17 % | 75 % | – | – | – | – |
Masinghe [8] | 1974–2003 | 53 | 9 y | 46.3 % | 71.7 % | – | 58.3 % | 72.8 % | – |
Rueth [15] | 2000–2011 | 36 | 64 m | 100 % | 100 % | 100 % | 100 % | ||
Shannon [9] | 1975–2001 | 29 | 44 m | 31 % | 87.5 % | – | – | – | – |
Sohn [14] | 1990–2009 | 142 | 78 m | 81 % | 98 % | 93 % | |||
Vlastos [18] | 1951–1998 | 45 | 7 y | – | 85 % | 83 % | – | 75 % | 79 % |
Walker [16] | 1983–2006 | 750 | SEER | – | – | – | 63.5 % | 67.1 % | |
In a nationwide retrospective study from Korea, 142 patients could be identified that received treatment between 1990 and 2009 [14]. About two thirds of the population had N1 disease. Overall, 23 % underwent ALND and 40 % received additional whole breast radiotherapy or mastectomy, respectively. There was a trend toward improved 10-year overall survival with whole breast radiotherapy (98 %) and mastectomy (93 %) compared to ALND alone (81 %, p = 0.061), but patients with N3 disease were overrepresented in the ALND-only subgroup and advanced nodal status emerged as the only significant prognostic factor. Interestingly, a matched cohort analysis is presented in the publication which suggests that for patients with N1 disease, survival is significantly better for axillary CUP than for breast cancer, while there was no significant difference for N2/N3 status.
The most recent publication is a retrospective study of 36 patients treated at MD Anderson Cancer Center between 2000 and 2011 [15]. Most of the patients had N1 disease, and more than 90 % had a breast MRI to rule out an occult breast tumor. Breast conservation was performed in 75 % of the patients, and 92 % had radiotherapy to the breast/chest wall and 77 % to the supra-/infraclavicular lymph node area. Almost all patients received systemic therapy, mostly in the neoadjuvant setting. The overall survival with a median follow-up of 64 months was 97.2 % with only one distant recurrence, ultimately resulting in the death of the patient. There were no local or regional recurrences.
The largest study is an epidemiological report from the Surveillance, Epidemiology, and End Results (SEER) database including 750 patients [16]. During the timeframe of the study, there was a significant decline in the use of mastectomy from 50 % before 1998 to 42 % during 1998 and afterward. Walker et al. showed that both mastectomy and whole breast radiotherapy conferred a significant overall survival benefit over ALND or selective lymph node excision alone, while there was no significant difference in 10-year cancer-specific survival between patients receiving mastectomy or whole breast radiotherapy. In a multivariate analysis, negative estrogen receptor status, 10 or more involved lymph nodes and resection of 10 or less lymph nodes emerged as negative prognostic factors.
9.1.1.4 National and International Guidelines
The NCCN guidelines for CUP and breast cancer offer recommendations on diagnostic and therapeutic management of patients with axillary CUP. Mastectomy and whole breast radiotherapy with or without nodal irradiation are being presented as equal alternatives. The use of breast MRI is recommended due to its higher sensitivity compared to mammography and ultrasound.
The German working group for Gynecologic Oncology (AGO) also recommends the use of breast MRI in patients with axillary CUP in their yearly updated guidelines. In contrast to the NCCN guidelines, mastectomy is not advised, especially not in patients with a negative breast MRI [17]. There is no clear recommendation for or against whole breast radiotherapy, although the guidelines state that there should be a discussion with the patient about its possible benefits in terms of local control and survival. This is also inconsistent with the NCCN recommendations.
9.1.1.5 Radiotherapy Treatment
Due to the limited evidence available for axillary CUP, most treatment recommendations are derived from publications on breast cancer. Radiotherapy dose, fractionation, and target volume were not consistently reported in the abovementioned studies. In the studies giving at least some treatment information concerning radiotherapy [5–7, 18], treatment was performed in analogy to breast cancer. The most common dose and fractionation regimen to the whole breast was 50–50.4 Gy in 25–28 fractions, although hypofractionation (40 Gy in 15 fractions) was used in a minority of patients in two publications [5, 6]. Barton et al. provided a subgroup analysis and were unable to show a benefit of a dose escalation beyond 50 Gy (or 40 Gy in hypofractionation) regarding locoregional recurrence and recurrence-free survival, but this analysis was limited to a total of 31 CUP patients [5]. Since the publication of the 10-year follow-up results of the major hypofractionation trials, hypofractionation has been acknowledged as a treatment standard in most national and international guidelines for the treatment of breast cancer [19, 20].
In breast-conserving treatment of breast cancer, the application of a boost dose of 10–16 Gy in 5–8 fractions to the tumor bed is commonly used and reduces the local recurrence risk by about 50 % without an effect on survival [21]. In the treatment of axillary CUP, there usually is no indication for the application of a boost, albeit there is residual tumor and a reexcision is not possible.
In the case of a complete axillary dissection, the axillary lymphatic levels I–III are typically not included in the clinical target volume in breast cancer although especially level I receives an incidental dose due to the close proximity to the breast. Indications to include the axilla in the clinical target volume are an incomplete axillary dissection, residual tumor with no possibility of reexcision, and advanced nodal disease, typically N3 with a ratio of involved to uninvolved lymph nodes over 0.75. In patients with 4 or more involved lymph nodes, the periclavicular lymphatic drainage should be included in the clinical target volume. Two large randomized trials have recently suggested that there might be a benefit of locoregional radiotherapy treatment (including the periclavicular and the parasternal lymphatic drainage) on locoregional recurrence and also distant recurrence in patients with breast cancer and N1 disease as well as selected patients with negative nodes and high-risk features, but these results have still not been fully published. Thus, a definitive conclusion on the role of locoregional radiotherapy in N0/N1 disease cannot be made [22].
Postmastectomy radiotherapy (PMRT) has mostly been recommended in breast cancer patients with at least 4 involved lymph nodes, but recently, an update of the EBCTCG meta-analysis showed that the benefit of PMRT is similar in patients with 1–3 involved lymph nodes with an absolute improvement in breast cancer-specific survival of about 8 % after 20 years [23].
Treatment of the chest wall and the regional lymphatic drainage in breast cancer patients is typically delivered using normofractionation although some patients after mastectomy and locoregional radiotherapy were included in the hypofractionation trials [19].
Axillary CUP with adjuvant breast irradiation with or without inclusion of the supraclavicular fossa can be adequately treated with 3D conformal radiotherapy in most cases. Intensity-modulated radiotherapy (IMRT) can be useful in the case of special anatomic situations, especially pectus excavatum [24]. Figure 9.1 shows a comparison of 3D conformal radiotherapy and IMRT in a sample case with an accentuated curvature of the thoracic wall.
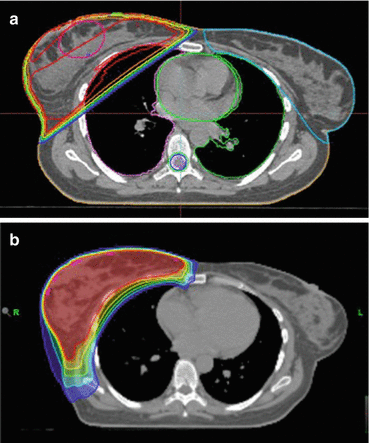

Fig. 9.1
Comparison of 3D conformal radiotherapy (a) and IMRT performed as a helical tomotherapy (b) in a patient with special anatomy. IMRT offers improved sparing of the right lung
IMRT might also be advantageous if the internal mammary chain is included in the target volume [25].
9.1.1.6 Conclusions
The evidence on the use of radiotherapy in axillary CUP is limited. Many retrospective studies show that whole breast radiotherapy is a safe alternative to mastectomy and that there is a significant benefit on local control and in some studies on survival compared to axillary dissection alone. However, this benefit might be related to the low quality of imaging and the suboptimal systemic treatment used in these studies. Nevertheless, there are no data that support the omission of the local treatment of the breast (whole breast radiotherapy or mastectomy) in the context of a negative breast MRI as suggested by the AGO guidelines. In breast cancer, prospective studies have shown that breast MRI does not result in a lower rate of reexcisions and does increase the likelihood of performing a mastectomy.
Keeping in mind the negative impact of mastectomy on quality of life, especially body image and sexual functioning in breast cancer patients [26], the low rates of severe late toxicity with modern radiotherapy techniques, and the comparable survival rates, whole breast radiotherapy offers a reasonable alternative to mastectomy in patients with axillary CUP. In the absence of clinical data on the role of PMRT and locoregional radiotherapy in patients with axillary CUP, these indications should be based on the available recommendations for breast cancer patients with involvement of the axillary lymph nodes.
9.1.2 Cervical CUP
Cervical CUP is an uncommon entity and accounts for only about 5 % of the malignancies in the head and neck region. Histologically, most cases reveal lymph node metastasis of a squamous cell carcinoma, although in a minority a diagnosis of undifferentiated carcinoma, adenocarcinoma, or malignant melanoma is made [27].
Concerning risk factors, a German retrospective analysis showed that 80 % of patients were current or former smokers [28]. HPV status was also assessed, and about half of the patients had evidence of HPV-DNA and/or were positive on p16-INK4A immunohistochemistry. Several publications could show that p16 and/or HPV positivity predicts oropharyngeal location of previously undetected primaries [29, 30]. Similarly, positivity for EBV or evidence of lymphoepithelial carcinoma can indicate a tumor location in the nasopharynx.
Before diagnosing a cervical CUP, patients should receive a systematic staging consisting of panendoscopy, CT, and/or MRI of the head and neck region and CT or x-ray of the chest. FDG-PET can further increase the sensitivity of diagnostic imaging, but its additional value decreases if panendoscopy and other imaging modalities have already been utilized [31].
9.1.2.1 General Management
Main approaches to cervical CUP are primary surgery with neck dissection followed by adjuvant radiotherapy compared to lymph node biopsy followed by definitive radio(chemo)therapy. Important prognostic factors are the extent of nodal disease [32–35], extracapsular extension of nodal metastases [32, 35–38], and the performance status at first diagnosis [33, 39].
Several articles have addressed the role of the extent of surgery in patients with cervical CUP. For example, Christansen et al. [40], Hauswald et al. [38], and Issing et al. [41] found a significantly lower rate of nodal relapses in the neck after more extensive surgery, i.e., neck dissection and in the latter two publications tonsillectomy versus biopsy before radiotherapy. Nonetheless, a systematic review including 18 retrospective studies with a total of 1726 patients showed no significant influence of the extent of surgery on survival, although the 5-year overall survival was numerically higher in patients with more extensive surgery [42]. Possible reasons for the positive results of the previously mentioned trials include a selection bias due to comorbidities or technical irresectability precluding a surgical approach and underestimation of the clinical tumor stage in patients undergoing definitive radiotherapy.
In patients with residual disease after definitive radio(chemo)therapy, a salvage neck dissection can be discussed [43].
9.1.2.2 Radiotherapy Target Definition
In cervical CUP, radiotherapy targets not only the areas of nodal involvement but also potential sites of occult mucosal disease. Historical series using surgery alone have shown that metachronous emergence of a primary tumor in the mucosa is a relevant endpoint in cervical CUP. In a publication by the Danish DAHANCA group, mucosal failure after 5 years occurred in 54 % of patients treated with surgery alone compared to 23 and 13 % of patients receiving radiotherapy to the neck alone or to the neck and the mucosa, respectively [33]. The most common location of an emerging primary tumor was the oropharynx in about half of the patients followed by the lung and the esophagus.
A subgroup that might be managed without adjuvant radiotherapy are patients with N1 disease without extracapsular extension after neck dissection since relapse rates are low, and the long-term outcome is favorable for these patients [36, 44].
An important area of debate is if the contralateral neck should be irradiated in patients with unilateral disease since bilateral radiotherapy increases both acute and late toxicities [37, 45]. Several publications reported that nodal or mucosal relapses are not significantly more common after unilateral radiotherapy (see Table 9.2). It is important to state that most of the patients in these series received adjuvant radiotherapy after neck dissection. About 40 % had N1 or N2a disease, whereas definitive radiotherapy and negative prognostic factors like N3 disease were usually more common in the bilateral treatment groups, representing a possible selection bias [39, 46]. Grau et al. provided results for 277 patients mostly treated by definitive bilateral radiotherapy including potential mucosal tumor sites after lymph node biopsy [33]. After adjustment for clinical covariates, unilateral radiotherapy was associated with a relative risk of 1.9 for locoregional failure, while the difference in disease-specific survival was not statistically significant. A study by Reddy and Marks provides treatment outcomes by nodal stage. While there was no significant difference between unilateral and bilateral radiotherapy for N1 patients, bilateral radiotherapy significantly increased nodal and mucosal control rates in patients with N2/N3 stage [45]. Survival was similar in both groups. Thus, unilateral adjuvant radiotherapy represents a treatment option mainly for patients with limited nodal disease after initial neck dissection [44].
Table 9.2
Studies comparing unilateral (UL) and bilateral radiotherapy (BL) in cervical CUP
Author | Study period | n | Follow-up | LC UL/BL | DFS UL/BL | OS UL/BL | Details |
|---|---|---|---|---|---|---|---|
Fakhrian [37] | 1988–2009 | 65 | 64 m | n.a. | 58 %/62 % | 44 %/49 % | 79 % ND 87 % N1–N2b |
Grau [33] | 1975–1995 | 277 | 137 m | 27 %/51 % (p = 0.05) | n.a. | 28 %/45 % (DSS) | 2 % ND 62 % N1–N2b |
Ligey [39] | 1990–2007 | 95 | 3.3 y | 66 %/75 % | 22 %/23 % | 83 % ND 67 % N1–N2b 45 % Chemo | |
Perkins [46] | 1989–2008 | 46 | 4.6 y | n.a. | n.s. | 77 % overall | 80 % ND 76 % N1–N2b |
Reddy [45] | 1974–1989 | 52 | n.a. | 66 %/73 % | 53 %/47 % | n.a. | 60 % ND 51 % N1–N2b |
There have been some publications addressing the question of elective mucosal coverage in cervical CUP. In patients without involvement of lymph node level III, exclusion of the hypopharynx and larynx still resulted in a 5-year mucosal control rate of 100 % [34]. Based on their data from 87 patients, Glynne-Jones et al. suggested to exclude the nasopharynx from the elective irradiation target volume. However, the nasopharynx was the second most common site of mucosal relapse in 4 out of 19 patients with omission of radiotherapy to this area [47]. Mourad et al. treated 68 patients with limited field radiotherapy including the oropharynx, the retropharyngeal nodes, and the bilateral neck. The locoregional control rate was 95.5 % after 3.5 years with one mucosal and two nodal recurrences.
It is crucial to remember that most of the abovementioned results were achieved with outdated imaging and radiotherapy techniques. With the combination of modern imaging modalities, parameters allowing for a prediction of the location of an occult primary tumor such as p16-INK4A, and more advanced radiotherapy techniques, individualization of the postoperative treatment volume will be an important issue in the near future.
9.1.2.3 Combined Chemoradiotherapy
In definitive radiotherapy of locally advanced head and neck squamous cell carcinomas (laHNSCC), a clear benefit of concurrent chemotherapy has been shown in terms of local control and overall survival [48]. In the setting of adjuvant radiotherapy for laHNSCC, concomitant chemotherapy is typically recommended in patients with adverse factors such as residual tumor and extracapsular extension of lymph node metastases [49].
In cervical CUP, the indication for combined chemoradiotherapy is usually based on the same factors, but specific evidence is limited.
Argiris et al. analyzed outcomes of 25 patients treated on different prospective protocols with combined chemoradiotherapy for cervical CUP [50]. The treatment consisted of split-course radiotherapy with a 5-FU and hydroxyurea backbone. Two trials applied induction chemotherapy, two trials tested simultaneous paclitaxel, and one trial used simultaneous cisplatin with hyperfractionated-accelerated radiotherapy. 56 % of patients had a neck dissection before treatment, and about 70 % of patients had N2a/N2b disease. The outcome was very favorable, only one patient developed a locoregional nodal relapse, and 5-year progression-free and overall survival were 75 and 83 %. There was one treatment-related death. Nutritional support via gastrostomy beyond 1 year after treatment was required in 16 % of patients. Xerostomy was reported in 44 % of cases.
Shehadeh et al. reported their experience on adjuvant platinum-based chemoradiotherapy in 37 patients with cervical CUP after neck dissection [51]. Most patients received 3 concurrent cycles of cisplatinum. The stage was N1–N2b in about 60 % of patients. There were only 2 regional failures, both in patients with N3 disease and extracapsular extension. Four patients developed distant metastases. Toxicity was acceptable with 3 cases of esophageal stricture. There were no long-term feeding tube dependencies.
In a retrospective, single-institution study, Chen et al. failed to show a benefit of the addition of concurrent cisplatin to radiotherapy in 60 patients with cervical CUP [52]. Cisplatin improved neither local control nor overall survival. 70 % of patients were treated in an adjuvant setting after neck dissection, and about 80 % had N1–N2b disease. Both acute and chronic toxicities were significantly higher with the addition of cisplatin.
Ligey et al. and Hauswald et al. provided subgroup analyses in their retrospective publications that also showed no additional benefit with concurrent chemotherapy [38, 39]. In the retrospective study of Fakhrian et al., there was a trend for improved overall survival (p = 0.051), but recurrence-free survival was unchanged [37].
In summary, the rationale for the addition of concurrent chemotherapy to radiotherapy for cervical CUP is scarce. Available evidence suggests that toxicity is increased, while no study so far has shown a benefit in terms of local control or survival. Nonetheless, due to the low level of evidence with multiple retrospective publications and one pooled analysis of several prospective single-arm studies and the heterogeneity of chemotherapy and radiotherapy regimens, the risk of bias is high. We believe that concurrent chemotherapy should be used in patients with an acceptable performance status and without significant comorbidities in high-risk situations, especially in the case of advanced nodal stages with extracapsular extension and when definitive radiotherapy is planned.
9.1.2.4 Intensity-Modulated Radiotherapy (IMRT) in Cervical CUP
Radiotherapy delivery and planning technique have undergone a significant evolution during the past decades. With the growing use of IMRT, many publications have addressed its application in head and neck oncology. It allows for a substantial increase in conformality of dose delivery and thus an optimized target volume coverage. On the other hand, the generation of sharp dose gradients improves sparing of surrounding normal tissue.
An example of a bilateral treatment plan is shown in Fig. 9.2.
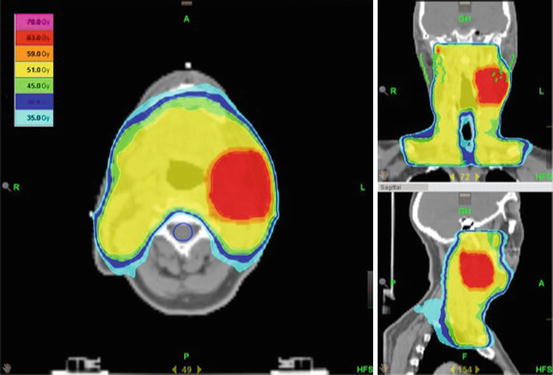

Fig. 9.2
Treatment of the bilateral neck and the mucosa in a patient with cervical CUP. The patient was initially treated with a neck dissection and had involvement of level II on the left side with extracapsular extension. Dose prescription was 66 Gy to the boost area (level II) and 54 Gy to the uninvolved neck and mucosa in 30 fractions. Optimal sparing of the parotid glands (green), the spinal cord (blue), and the trachea and cervical esophagus was provided
The randomized phase III PARSPORT trial comparing 3D conformal radiotherapy to IMRT in head and neck squamous cell carcinoma patients showed that the reduction of radiation dose to the parotid glands through the use of IMRT translated into a significant reduction of xerostomia and salivary flow impairment and an improvement in the related quality of life domains [53]. Grade 2 or worse xerostomia at 1 year after treatment was reduced from 74 to 38 %. Chen et al. retrospectively analyzed dosimetric parameters for 51 cervical CUP patients treated with 3D conformal or intensity-modulated radiotherapy and also found a significant reduction of the radiation dose to the parotid gland on the noninvolved side of the neck in a similar magnitude as in the PARSPORT results [54]. This also led to a reduction of significant late xerostomia from 58 to 11 %. Gastric feeding tube dependency was 42 % versus 11 %, while the overall incidence of grade 3 or worse toxicity was 63 % versus 29 %. Similar results were published by other groups [55].
The only study to show an impact of radiotherapy technique on clinical outcome was the publication by Ligey et al. 95 patients who were treated by 2D radiotherapy using either wedged lateral fields or parallel opposed fields with an anterior field for the lower neck or CT-planned 3D conformal radiotherapy/IMRT were studied retrospectively [39]. On multivariate analysis, radiation technique was an independent prognostic factor for both local control and overall survival.
Recently, Janssen et al. published their experience with a risk-adapted approach of radiotherapy with IMRT and 3 dose levels for different risks of involvement according to the nodal involvement [56]. Overall survival, disease-free survival, and nodal control were 76, 81, and 93 % at 3 years. There were no late toxicities of grade II or higher.
Most reports of IMRT have shown very favorable clinical results (see Table 9.3); nonetheless, all of them are small, single-institutional studies, and the better outcome compared to historical reports might also represent a change in the underlying risk factors as well as changes in surgical approaches and chemotherapy use. While toxicity appears to be acceptable and significantly lower compared to 3D conformal radiotherapy, reports of increased rates of esophageal stenosis with IMRT and concurrent chemotherapy treatment warrant further investigation [57].
Table 9.3
Experience with IMRT in cervical CUP
Author | Study period | n | Follow-up | LC | DFS | OS | Details |
|---|---|---|---|---|---|---|---|
Chen [54] | 2001–2009 | 24 | 29 m | 92 % | – | 87 % | 63 % Chemo, 70 % ND, |
Janssen [56] | 2006–2012 | 28 | 30.5 m | 93 % (neck), 100 % (mucosa) | 81 % | 76 % | 71 % Chemo, 71 % ND |
Frank [92] | 1998–2005 | 52 | 3.7 y | 94.2 % | 88 % | 81 % | 27 % Chemo, 25 % ND |
Sher [57] | 2004–2009 | 24 | 2.1 y | 100 % | 92 % | 92 % | 92 % Chemo, 13 % ND |
Shoushtari [93] | 2002–2008 | 27 | 41.9 m (surviving) | 88.5 % (neck) | 85.2 % | 70.9 % | 30 % Chemo, 80 % ND |
Klem [94] | 2002–2005 | 21 | 20 m | 90 % | – | 85 % | 76 % Chemo, 67 % ND |
Madani [55]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|