Fig. 6.1
Encryption with a hash function
The patient data were divided into personal data (name, date of birth, medical record number, etc.) and disease data (tumor location, T-factor, N-factor, M-factor, pathological data, treatment method, etc.). The personal data were encrypted as the hash value, and data packages consisting of the personal data encrypted as the hash value and the disease data were exported from each institution to the data center. An examination of the hash data enables double registrations to be identified and patient outcome to be followed.
The ethics committee of the Japan Esophageal Society reviewed and approved the registration project and the use of “hash function” encryption. The registration project was also reviewed and approved by the institutional review board of each institution.
6.3.3 Certification of the Registration Project
In the era of the Japan Society of Esophageal Diseases, that is, the former name for the Japan Esophageal Society, the membership consisted of institutions. In the era of the Japan Esophageal Society, however, the membership is comprised of doctors. Basically, the institutions where the members work should be requested to register esophageal cancer cases, and cooperative institutions for the registration project were approved and certificates were issued to 456 institutions in February 2008 (Fig. 6.2).


Fig. 6.2
Certificate of approval for institutional registration issued by the Registration Committee for Esophageal Cancer of the Japan Esophageal Society
6.3.4 Preparation of Registration Sheets
6.3.5 Trial of the New Registration Project
Next, each member of the Registration Committee for Esophageal Cancer tested the new registration system using the “hash function” encryption (Fig. 6.3). A CD-R containing the recording software and the “hash function” encryption software and a return CD-R, on which the data would be recorded, were sent to each member. Each member recorded the data package of the encrypted personal data as a hash value and the disease data of patients who were treated in 2001 and then returned the CD-R back to the data center. No difficulties were encountered in the mailing of the CD-R, and that the registration and encryption software worked correctly. Moreover, the data center succeeded in a similar analysis of data collected as the Comprehensive Registry of Esophageal Cancer in Japan, 2000 [10], and the functionality of the new analyzing software was confirmed.
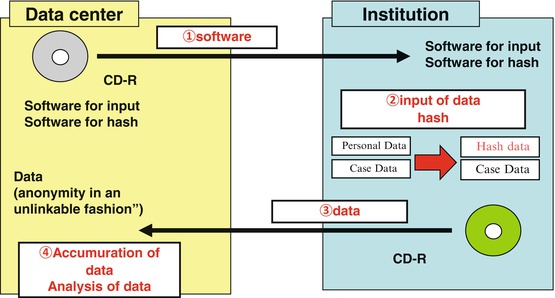

Fig. 6.3
Outline of data collection
6.4 Resumption of the Registration Project
To resume the registration project, many problems were resolved, one by one. In March 2008, the new registration project was started for patients who had been treated in 2001. The CD-R containing the recording software and the “hash function” encryption software and the return CD-R, on which the data would be recorded, were sent to the approved institutions. A website for the registration project was created on the homepage of the Japan Esophageal Society. As of August 19, 2008, a total of 3,940 cases from 241 institutes (52.9 %) were registered.
6.5 Publication of the Resumed Reports
The committee members reviewed the analyzed results of the registered data, and the Comprehensive Registry of Esophageal Cancer in Japan, 2001, which was published on March 12, 2009, was sent to the approved institutions (Fig. 6.4). The Comprehensive Registry included 76 tables and 16 figures and showed the current status of esophageal cancer treatment in Japan. Twenty-three selected tables and 16 figures were published in Esophagus (Vol. 6, pages 95–110) [2], the official journal of the Japan Esophageal Society, to ensure wide and easy access to the latest information regarding esophageal cancer treatments (Fig. 6.5).



Fig. 6.4
Front cover of the Comprehensive Registry of Esophageal Cancer in Japan, 2001

Fig. 6.5
Excerpted version of the Comprehensive Registry of Esophageal Cancer in Japan, 2001, published in Esophagus (Vol. 6, pages 95–110)
6.6 Next Year Registration
After problems with the registration system used for the cases registered in 2001 were improved, a registration project for cases treated in 2002 was started on March 26, 2009. As of August 31, 2009, a total of 4281 cases from 222 institutes (48.7 %) had been registered. The committee members reviewed the analyzed results of the registered data, and the Comprehensive Registry of Esophageal Cancer in Japan, 2002, was published on March 1, 2010 [11], and sent to the approved institutions.
6.7 Problems Arising During the First 2 Years
Although the new registration system required “anonymity in an unlinkable fashion,” some institutes very nearly returned data packages containing non-encrypted personal data and disease data to the data center. Although the “anonymity in an unlinkable fashion” step may seem laborious, members must understand that this step is indispensable for the continuation of the registration project.
The number of institutes that submitted CD-Rs to the data center was about 50 % of the total number of approved registration institutes. To grasp the real status of esophageal cancer treatment in Japan, more institutes need to return CD-Rs on which their activities have been recorded. The number of items on the registration forms was reduced, compared with the old registration form used for cases in 2000, to lighten the workload of the doctors in charge of registration.
6.8 Summary of the Comprehensive Registry of Esophageal Cancer in Japan, 2001–2006
We summarized the Comprehensive Registry of Esophageal Cancer in Japan, 2001–2006 [2, 11–15]. A total of 28,487 cases were registered from a total of 1,352 institutions in Japan. As for the histologic type of cancer according to biopsy specimens, squamous cell carcinoma and adenocarcinoma accounted for 88.7–92.9 % and 2.4–3.9 %, respectively. Regarding the clinical results, the 5-year survival rates of patients treated using endoscopic mucosal resection, concurrent chemoradiotherapy, radiotherapy alone, chemotherapy alone, and esophagectomy were 80.0–87.7 %, 19.3–26.4 %, 15.1–30.0 %, 1.7–8.6 %, and 42.6–50.9 %, respectively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree