Study/author/year
No. patients
Sites/no. pts. OP/OC/HP/L
Stage (UICC)
Fractionation schedule
OTT (weeks)
Total dose in tt.-arms
LC/LRC (%)
PFS (%)
OS (%)
Acute toxicities
Late morbidities
Pinto et al. (1991) [19]
56
56/–/–/–
III/IV
HFX: 1.1 Gy bid
6.5
HFX: 70.4 Gy
LC: 84 % p = .02
25 % p = .08
8 % @ p = 0.03
Same degree but earlier onset for HFX
No differences between tt.-arms
56
56/–/–/–
ditto
CFX: 2.0 Gy qd
6.5
CFX: 66.0 Gy
LC: 64 % @ 3.5 yrs
7 % @ 3.5 yrs
27 % 3.5 yrs
EORTC 22791
180
180/–/–/–
III
HFX: 1.15 Gy bid
7.0
HFX: 80.5 Gy
LC: 59 % p = .02
Not stated
39 %; p = .08
Diffuse mucositis: HFX: 67 %
No differences between tt.-arms
Horiot et al. (1992) [21]
176
176/–/–/–
ditto
CFX: 2.0 Gy qd
7.0
CFX: 70.0 Gy
LC: 40 %; @ 5 yrs
Not stated
30 % @ 5 yrs
CFX: 49 %; p = .01
RTOG 09–03
211
160/26/28/49
II/III/IV
HFX: 1.2 Gy bid
7.0
HFX: 81.6 Gy
LRC: 54.4 % @ 2 yrs p = .045
37.6 % @ 2 yrs
54.5 % @ 2 yrs
All patients in the AFX treatment groups had enhanced acute toxicities
Feeding tubes @ 5 yrs in survivors: 4.8 % for HFX vs. 13 % for SFX, no differences in other late effects
274
165/29/40/40
ditto
Split-ACF: 1.6 Gy bid
5.0
Split-ACF: 67.2 Gy
LRC: 47.5 % @ 2 yrs
33.2 % @ 2 yrs
46.2 % @ 2 yrs
268
165/24/39/40
ditto
Conc. Boost: 1.8 + 1.6
6.0
Conc. Boost: 72 Gy
LRC: 54.5 % @ 2 yrs p = .05
39.3 % @ 2 yrs
50.9 % @ 2 yrs
268
159/31/34/44
ditto
CFX: 2.0 Gy qd
7.0
CFX: 70.0 Gy
LRC: 46 % @ 2 yrs
31.7 % @ 2 yrs
46.1 % @ 2 yrs
DAHANCA 6&7
Overgaard et al. (2003) [24]
730
213/71/–/466
II/III/IV
ACFX: 2.0 Gy qd 6×/week
5.6
ACF 66-68 Gy
LRC: 70 % @5 yrs, p = .0005
73 % @ 5 yrs
52 % @ 5 yrs
Enhanced acute toxicities
p < .0001
No differences p = .16
755
222/62/–/442
ditto
CFX: 2.0 Gy qd 5×/week
6.6
CFX 66–68 Gy
LRC: 60 % @ 5 yrs
66 %; p = .01
50 %; n.s.
CAIR
Skladowski et al. (2013) [25]
173
56/16/17/83
II/III/IV
CAIR: 2.0 Gy qd 7 days/week
5.3–5.7
CAIR 66.6–72.0 Gy
LRC: 63 %/60 % @ 5/10 yrs
Not stated
40 %/25 % @ 5/10 yrs
Confluent mucositis 3°: 89 % vs. 86 %
Only 6 % for both tt.-arms
172
61/13/15/82
ditto
CB: 2.0 Gy qd 5 days/week
5.3–5.7
CB: 66.6–72.0 Gy
LRC: 65 %/60 % @ 5/10 yrs
Not stated
44 %/25 % @ 5/10 yrs
CHART
Dische et al. (1997) [29]
552
141/79/53/224
II/III/IV
CHART: 1.5 Gy td 12 days
1.8
CHART: 54.0 Gy
LRC: 45 % @ 5 yrs
42 % @ 4 years
40 % @ 5 yrs
73 % confluent mucositis
Less severe late reactions
366
98/47/34/170
ditto
CFX: 2.0 Gy qd
6.6
CFX: 66.0 Gy
LRC: 44 % @ 5 yrs; n.s.
40 % @ 4 years; n.s.
40 % @ 5 yrs
43 % confluent mucositis
EORTC 22851
Horiot et al. (1997) [30]
257
153/38/–/32a
II/III/IV
ACFX: 28.8 Gy tid, 14 d. split 72 Gy tid
5.0
ACFX: 72.0 Gy
LRC: 59 % @ 5 yrs; p = .02
Not stated
26 % @ 5 yrs
OMRa @ 6 weeks: 70 %
SAE-free @ 5 yrs: 53 %
255
153/38/–/32a
ditto
CFX: 2.0 Gy qd 5×/week
7.0–8.0
CFX: 70.0 Gy
LRC: 46 % @ 5 yrs
Not stated
27 % @ 5 yrs
34 %
86 %
GORTEC
Bourhis et al. (2006) [31]
137
107/19/7/4
III/IV
ACFX: 2.0 Gy bid
3.2
ACFX: 64.0 Gy
42 % @ 6 yrs
No difference in both tt.-arms
20 % @ 5 yrs
Increased dermatitis 3°; each p < .0001
No differences for both treatment arms
129
98/17/8/6
ditto
CFX: 2.0 Gy qd 5×/week
7.0
CFX: 70.0 Gy
27 % @ 6 yrs p = .009
17 % @ 5 yrs n.s.
A selection of pivotal trials using AFX represents the experiences gained by the radiotherapeutic community worldwide. A small RCT of Pinto et al. (1991) treated 98 stage III/IV OPC patients using bid 1.1 Gy with interfraction intervals of 6 h up to 70.4 Gy vs. qd 2.0 Gy up to 66 Gy total dose, respectively. It could be shown that HFX was superior in terms of local control (LC; 84 % vs. 64 %, p = .02) and OS (27 % vs. 8 %, p = .04) at 3.5 year follow-up [19]. Acute mucosal and skin toxicities were similar but had an earlier onset with HFX. Late morbidity was not different between both arms.
A large 3-arm RCT was carried out by Sanchiz et al., 1990, in 859 patients with LAD HNSCC [20]. In the first treatment arm, 299 patients were treated with SF-RTX of weekly 5 × 2.0 Gy up to 60 Gy. In the second arm, 282 patients received bid 1.1 Gy with a fraction interval of at least 3 h up to a total dose of 70.4 Gy, and the third treatment arm included 300 patients with 5 × 2.0 Gy up to 60 Gy in 6 weeks OTT with concomitant 5-FU of 250 mg/m2 every other day. The acute mucosal and skin toxicity was moderate with 10 % grade 3 mucositis in the combined arm. Late morbidity was confined to moderate xerostomia in 42 %, bone necrosis in 11 % and cervical fibrosis in 19 % of all patients. Mean OS for group A (SFX) was 38.2 months vs. 84.0 months (p < .001) for group B (AFX). This study from the 1980s was hypothesis-generating for the development of subsequent RCT on AFX and concurrent CTX.
A large 2-arm trial investigating HFX was executed by the former EORTC Radiotherapy Group (Trial 22791) in 356 stage III OPC patients with fractions of bid 1.15 Gy vs. qd 2.0 Gy up to total dose levels of 80.5 Gy vs. 70.0 Gy, respectively [21]. At 5 years, an LRC benefit of 19 % (59 % vs. 40 %, p = 0.02) could be shown. A difference in acute toxicity was exclusively observed for grade 3 mucositis, which was dominant in the HFX-treatment arm with 66.5 % vs. 49 %. Late morbidity was not different between the two study arms.
Fu et al. published the by far largest trial addressing altered fractionation (RTOG 90–03) with a recruitment of 1073 stage II/III (≈30 %) and IV (≈70 %) patients, who were submitted to SFX, HFX, ACFX with split and moderately ACFX with concomitant boost (CB) during the fifth and sixth weeks of treatment by introducing a second fraction of 1.5 Gy in the afternoon [22]. The major involved sites were oropharynx (OP) ≈60 %, larynx ≈17 %, hypopharynx (HP) ≈13 % and oral cavity (OC) ≈10 %. The results at 2 years showed a statistically improved LRC for HFX (p = .045) and ACFX with CB (p = .05), however, no impact on disease-free (DFS) and OS. As in all AFX-trials the acute toxicities were enhanced, in some instances leading to treatment interruptions of 3–5 days. Late morbidities at 2 years of the experimental treatment arms were not different from those of the controls. An update of this trial with a maximum follow-up of 15 years reported by Beitler et al. 2014, showed only HFX to be superior to SFX in terms of a HR reduction for LRC of 21 % (HR: 0.79; p = .05) and for OS of 19 % (HR: 0.81) at 5 years [23]. Late morbidities grade 3–5 including use of feeding tubes were not statistically different in the whole study population. However, surviving patients at 5 years experienced with 13 % vs. 4.8 % for the ACFX-regimens a higher feeding tube dependence compared with HFX.
Selected Phase-III RCTs for Moderately Accelerated Fractionation (ACFX)
The DAHANCA 6 and 7 trials addressed in 1.485 patients the question of a superiority of a modest ACFX regimen of 66–68 Gy in 5.6 weeks vs. SFX using the same total dose with an OTT of 6.6 weeks in moderately advanced (ca. 50 % stage III/IV) HNSCC with about two thirds of prognostic favourable laryngeal cancers. The experimental arm received six fractions of 2.0 Gy including Saturdays vs. five weekly fractions in the standard arm [24]. As a result of this moderate acceleration without a compromise in total dose the hazard of accelerated tumour clonogen repopulation, mainly during the weekends, could be minimized leading to an improvement of the LRC-rates by 10 % (70 % vs. 60 %; p = .0005). Moreover, primary tumour control was improved from 64 to 76 % (p = .0001) and voice preservation was successful in 80 % (ACFX) vs. 66 % (CFX; p = .007). DFS was also enhanced from 66 % to 73 % (p = .01), but not OS. The acute toxicities of ACFX were more pronounced; however, no differences in late morbidity were observed.
Another RCT in 345 patients compared a 7-days-a-week (“weekend-on”) ACFX regimen (CAIR = Continuous Accelerated IRradiation) with single fractions of 1.8 Gy against a 5-day concomitant boost (CB) regimen with 1.8 Gy qd on Monday, Wednesday and Thursday and bid 1.8 Gy on Tuesday and Friday (“weekend-off”) to the same total doses depending on the initial T-stage (T2 = 66.6–68.4 Gy, T2/3 = 70.2–72.0 Gy) in 37–40 fractions during 5.3–5.7 weeks with moderately advanced HNSCC (60 % stage IV) [25]. LC at 5 and 10 years for CAIR was 63 % and 60 %, respectively, or 65 % and 60 % for the CB. Corresponding OS-figures were 40 % and 25 % for CAIR vs. 44 % and 25 % for CB at 5 and 10 years, respectively. Acute confluent mucositis developed as the major symptom with CAIR (89 %) and CB (86 %). The 5-year late radiation morbidity was low with 6 % in both treatment arms. This study clearly showed that the “weekend-on” regimen can be skipped in favour of a “weekend-off” regimen without losing on tumour control or survival. Without chemotherapy (CTX), a moderate acceleration of the OTT with weekly doses of 12 Gy is beneficial for the patients and well tolerated by normal tissues and organs at risk at the head and neck region.
Selected Phase-II/III RCTs for Very Accelerated Fractionation (ACFX)
These regimens involve a radical reduction of the overall treatment time from 7 to 8 to about 2 weeks or even less. It needs three fractions per day and a substantial reduction of the total dose to prevent excessive acute mucosal and dermal reactions. A considerable number of studies have focused on very accelerated regimens in order to attain an enhanced tumour cell kill and also to avoid accelerated tumour clonogenic repopulation. The most intensive continuous course regime was done by Perachia et al. 1981, who treated 22 patients with three fractions of 2 Gy with 4 h intervals to 48–54 Gy within only 9–11 days [26]. This led to intolerable acute reactions including 60 % severe necrosis and fistulas and some deaths soon after treatment.
Svoboda et al. treated 59 patients with thrice daily fractions of 1.7–2.3 Gy with intervals of at least 3 h to a total dose of 50–55 Gy in 1.5–2.0 weeks. The 3 years OS of 44 % in LAD was notable; however, a rate of 15 % of late necrosis and stenosis also compromised the overall results [27].
Nguyen et al.1985, published on a rapid AFX in 178 patients with LAD HNSCC [28]. Patients in two cohorts were treated with initially 40 fractions of 0.9 Gy with 2 h interval up to 36 Gy during the first 5 days. After 2 weeks rest, a similar course was applied with reduced fields up to 72 Gy in an OTT of 24 days. For the second cohort the total dose was reduced to 60–66 Gy and the rest period extended from 2 to 4 weeks. At the end of these treatments, about two thirds of the patients had a complete remission; however, 56 % developed local recurrences during follow-up. The OS-rate at 2 years was only 13 %. The acute toxicity was severe in one third of the patients with extensive mucosal necrosis and bleeding. Also the rate of late complications with trismus, extensive cervical fibrosis and permanent laryngeal oedemas was enhanced. This indicates the critical role of an adequate interfraction interval as already mentioned above.
A pivotal RCT in terms of patients and acceleration was the CHART trial. This by far largest trial for Continuous Hyperfractionated Accelerated RadioTherapy (CHART) recruited at total of 918 patients for the experimental arm with thrice daily 1.5 Gy to a total dose of 54 Gy in 12 days versus the SFX using single doses of 2 Gy to a total dose of 66 Gy [29]. Despite a dose reduction from 66 to 54 Gy, the endpoints, e.g. LRC and OS, were not inferior for CHART. Acute toxicities, however, were more pronounced with CHART opposite to reduced late morbidities. This study clearly pointed out the considerable impact of repopulation as cause of recurrent disease in the absence of CTX or other tumour cell proliferation inhibitors.
An accelerated split-course RCT was investigated on an EORTC platform (EORTC 22851) in 512 patients with moderately advanced stage II–IV HNSCC [30]. Three fractions (tid) of 1.6 Gy were applied during 8 days to a dose of 28.8 Gy with a subsequent split of a fortnight. Thereafter the same schedule was continued with 27 fractions in 17 days to a total of 72 Gy in 5.2 weeks OTT. The SFX-arm used 35 fractions of 2.0 Gy in 7 weeks. The split-ACFX regimen was superior in terms of LRC with 59 % vs. 46 % (p = .02). However, OS showed no difference with 26 % vs. 27 %, respectively. For the split-ACFX arm, 70 % acute objective mucosal reactions vs. 34 %, respectively, and 5 % vs. 2 % life-threatening severe adverse events (SAE) were reported. Also late morbidity was significantly enhanced with only 53 % SAE-free survival vs. 86 % for SFX. Due to the high rates of acute and late radiation sequelae split course ACFX was no longer pursued.
The French collaborative head and neck group (GORTEC) carried out a RCT employing a very accelerated radiation regimen with a total dose of 62–64 Gy with bid 2 Gy in 31–32 fractions within 2.5 weeks vs. a SFX of 70 Gy with qd 2.0 Gy in 35 fractions in 268 patients [31]. The majority of patients (78 %) had OPC (≈78 %) and about 66 % UICC stage-IV tumours. With the very ACFX regimen the LRC rate could be significantly improved by 24 % at 6 years, whereas DFS and OS were not different in both treatment arms. Acute WHO grade III/IV mucositis was enhanced with very ACFX up to 90 % vs. 51 % for the control arm. No difference in late effects between the two treatment arms was observed.
Altered Fractionated Radiotherapy – Results from Meta-Analyses
The abundance of available data from RCTs as partly cited above qualify for a comprehensive meta-analysis based either on published hazard ratios (HR) and survival curves or on the integration of updated data sets from individual patients.
A meta-analysis based on published OS probabilities of 4792 patients showed no OS-benefit at 2 years and no prolongation of the median survival nor a significant HR reduction for ACFX vs. SFX, respectively. In contrast, HFX vs. SFX led to a significant OS benefit of 12 % at 2 years (n = 1523) corresponding to a median OS prolongation of 14.2 months and a 14 % reduction of the HR for death [32].
An international collaborative group of primary investigators agreed to establish a collaborative meta-analysis group, which provided individual patients’ data for AFX based on 7073 patients. This Meta-Analysis of Radiotherapy in Carcinomas of Head and Neck (MARCH) addressed AFX regimens like HFX and ACFX with or without (w/o) total dose reduction in comparison with SFX [33]. The majority of patients suffered from HNSCC of the OP and larynx in stage III and IV. Multiple endpoints like HRs for death and cancer/non-cancer death, LC, LRC, regional control (RC), metastatic control and corresponding survival figures were analysed (Figs. 9.1 and 9.2). The results at 5 years from four RCTs based on a total of 680 patients clearly favour HFX with an absolute OS-benefit of 8.2 % ± 2.6 % vs. 1.7 % ± 2.3 % and 2.0 % ± 1.7 % for ACFX w/o total dose reduction (p = 0.003), respectively. Cancer specific death was improved by 7.8 % ± 2.8 % in favour of HFX corresponding to a HR reduction of 22 % (95%CI: 10–32 %). HFX vs. SFX showed a benefit in PFS of 7.8 % ± 2.8 % (45 % vs. 36.9 %). LRC figures were also superior with 9.4 % ± 3.0 % at 5 years and interestingly also of 7.3 % ± 1.7 % for the ACFX without dose compromise (p < .0001). The HRs for death were reduced for HFX by 22 % (95%CI: 10–32 %) and for LRC by 24 % (95%CI: 11–34 %) as also for ACFX without dose reduction by 21 % (95%CI: 13–28 %). The benefit in LRC could exclusively be attributed to an improved LC with an overall relative HR reduction for the three treatment groups (HFX, ACFX w/o total dose reduction) by 23 % (95%CI: 17–29 %, p < .0001), but not to nodal control and DM. In terms of HRs for death a significant interaction with age after AFX compared with SFX was observed, which could not be established for sex, performance score, stage and site of the primary.
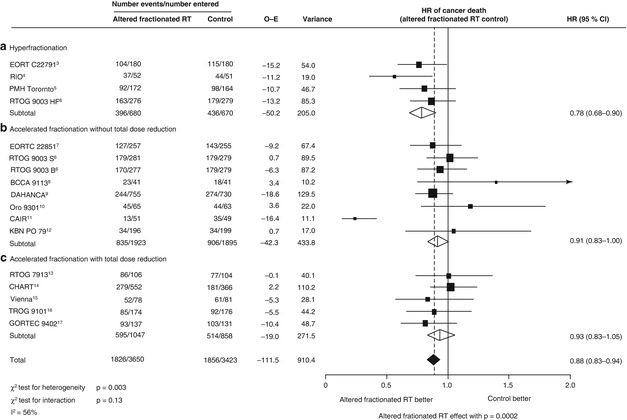
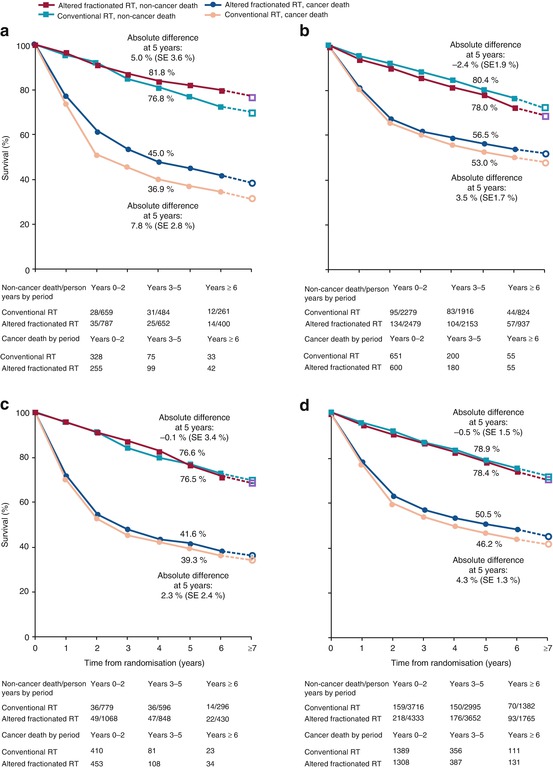

Fig. 9.1
Hazard ratio of head and neck cancer death with altered fractionated radiotherapy versus conventional radiotherapy. The centre of each square is the hazard ratio (HR) for individual trials and the corresponding horizontal line is the 95 % CI. The area of the square is proportional to the number of events from each trial. The broken line and centre of the black diamond is overall pooled HR and the horizontal tip of the diamond is the 95 % Cl. Open diamonds are the HR of different types of radiotherapy. BCCA British Columbia Cancer Agency, CAIR continuous accelerated irradiation, CHART continuous hyperfractionated accelerated radiation therapy, DAHANCA Danish Head and Neck Cancer Study Group, EORTC European Organisation for Research and Treatment of Cancer, GORTEC Groupe d’Oncologie Radiothérapie Tête et Cou, KBN Komiet Badan Naukowych (Committee for Scientific Research), 0-E observed minus expected, PMH-Toronto Princess Margaret Hospital, Toronto, RT radiotherapy, RTOG Radiation Therapy Oncology Group, TROG Trans-Tansman Radiation Oncology Group

Fig. 9.2
Non-cancer death and cancer death survival curves for all trials and for the three groups of trials according to the type of altered fractionated radiotherapy. (a) Hyperfractionation. (b) Accelerated fractionation without total close reduction. (c) Accelerated fractionation with total close reduction. (d) All three groups together. The slopes of the broken lines from year 6 to year ≥ 7 are based on the overall death rates in the seventh and subsequent years. RT radiotherapy
Take Home Message for Altered Fractionation, Part 1
Altered fractionation vs. standard fractionation led to a small but significant absolute OS benefit of 3.4 % at 5 years in patients with LAD HNSCC corresponding to a HR reduction for death of 8 %.
Hyperfractionation as a specific subtype of altered fractionation led to a highly significant absolute OS and LRC benefit of 8.2 % and 9.4 %, respectively, compared with standard fractionation corresponding to a HR reduction for death of 22 %, which was not observed for moderately and very accelerated fractionation regimens.
Hyperfractionation results in the best overall survival benefit compared with other altered fractionation regimens like accelerated fractionation w/o total dose compromise.
Hyperfractionation is a good alternative for definitive treatment of LAD HNSCC in patients who are not fit for concurrent chemoradiation or who have a high Charlson comorbidity score.
Take Home Message for Altered Fractionation, Part 2
Despite level IA evidence, hyperfractionation as a resource-intensive treatment is currently not in widespread use and limited to patients with interlaced target volume and critical organs at risk, which needs to be spared by biological means.
Hyperfractionated re-irradiation with bid 1–1.2 Gy to 66–70 Gy can be a treatment of choice in locally recurrent disease for a target volume >60 ml.
Accelerated fractionation shows the best efficacy for primary tumours and to a lesser extent for nodal disease.
Patients below the age of 70 years benefit from altered fractionation in terms of LRC and PFS. However, patients above 50 years of age have only marginal benefit in terms of overall survival from altered fractionation.
Concurrent Chemoradiation
Rationale and Caveats
The rationale for cCRTX is primarily the cooperation of cytotoxic effects leading to increased tumour cell kill and secondarily a dissociation of acute and late morbidities at the organs and tissues at risk, e.g. spinal cord, aiming at an improved therapeutic ratio [34–36]. Since the 80s of the last century the first RTX with concomitant CTX were carried out in LAD HNSCC [1, 3, 37]. At that period of time, drugs such as 5-FU, methotrexate, bleomycin, mitomycin C, cyclophosphamide, vincristine and vinblastine were employed. Many studies used multidrug and others single-drug combinations. In 1987 the first concurrent chemoradiation (cCRTX) with platinum/5-FU was introduced by Toohill et al. 1987 [38]. Since then, a large number of RCTXs have been successfully carried out. In recent years, Carboplatinum and Taxotere were also introduced in clinical trials in HNSCC [39–41]. Consequently, cCRTX has been continuously improved during the last decades and is still considered standard of care for LAD and functionally inoperable HNSCC. Despite a clear level IB evidence for improvements of the major endpoints like OS, LRC and DFS, in clinical practice, these benefits were often achieved at the expense of enhanced acute or late treatment related sequelae, e.g. functional deficits hampering an improved therapeutic ratio (Table 9.2).
Table 9.2
Selected pivotal randomized clinical trials with altered fractionated chemoradiation in LAD HNSCC
Study/author/year | Fractionationno. of pts. | Sites/no. pts. OP/OC/HP/L | Stage (UICC) | Fractionation schedule | OTT (weeks) | Total dose in tt.-arms | CTX drugs | LC/LRC (%) | Distant metastases rates (%)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|