Key points
- 1.
Women with gynecologic cancers can have significant complications from their cancer and/or treatments.
- 2.
Vaginal bleeding is usually controlled with packing although arterial embolization may be needed to control severe hemorrhage.
- 3.
Ureteral obstruction can be treated by diversion, either with ureteral stent placement, percutaneous nephrostomy, or surgery.
- 4.
Nearly all small bowel complications are initially treated with conservative management, but surgery may be required depending on the patient’s disease status and overall condition.
- 5.
Women with gynecologic cancers are at high risk of developing a venous thromboembolism (VTE), and prophylaxis is recommended in nearly all cases.
- 6.
Infectious complications must be treated aggressively in order to avoid delays in cancer treatment.
- 7.
Complications of disease and treatment often must be managed coincident with active cancer treatment.
Women with gynecologic cancers often experience complications associated with their primary disease process or from the cancer-directed treatment. In addition, many women have medical comorbidities, are obese, or are elderly, all of which may further complicate treatment decisions and therapy. Minimizing these problems requires the clinician to astutely evaluate the patient, be proactive in prevention strategies, and provide early identification and treatment of complications.
Complications of disease are, in fact, commonly the primary presenting symptom (chief complaint) of a gynecologic cancer. Common symptoms of gynecologic cancers include vaginal bleeding (cervical and endometrial cancers), urinary tract obstruction or fistulae (cervical cancer), or intestinal obstruction or weight loss (ovarian cancer). Although some complications have been discussed previously in this text, it seems appropriate to devote a chapter exclusively to complications of disease and therapy. Not all possible complications can be covered, and readers are referred to texts that expand on them; however, the more common complications and their management are discussed.
Disease–oriented complications
Symptoms caused by cancer, such as bleeding, urinary tract obstruction, fistula, and intestinal obstruction, are complications of the primary gynecologic cancer that usually need to be managed coincidentally with the cancer itself.
Hemorrhage
Bleeding from cervical or endometrial cancer is a common presenting symptom. Although bleeding is rarely severe, the acute management of hemorrhage may be required before cancer therapy can be undertaken. Patients who are bleeding should be initially assessed for hemodynamic stability. On rare occasions, the bleeding is so severe that the patient may be in hypovolemic shock. Immediate management should include securing venous access, blood volume replacement, and supportive care. When stabilized, the patient should be examined, and the source of the bleeding determined. Most commonly, massive hemorrhage results from an exophytic cervical cancer eroding into a small cervical or vaginal artery. Prolonged slow vaginal bleeding from an endometrial cancer or sarcoma may also result in a patient presenting with profound chronic anemia. Because the bleeding has been slow over a longer period, the patient has often adapted to the anemia and may be hemodynamically stable despite profound anemia. Biopsy should be performed to document the pathology, and the patient should be evaluated to make a clinical estimation of the extent (stage) of disease.
Control of an actively bleeding cervical lesion is usually accomplished with a vaginal pack applied firmly to the cervix, filling the entire vagina. Monsel’s solution (ferric subsulfate) may be put on the portion of the pack abutting the tumor. Soaking of the entire pack with Monsel’s solution should be avoided because it will desiccate the normal vaginal mucosa, making removal of the pack and subsequent pelvic examinations difficult. An indwelling Foley catheter should be placed in the bladder because pressure from the pack will usually obstruct the urethra resulting in urinary retention. The pack should be removed slowly 24 to 48 hours later, and the patient should be observed. Removal of the pack under anesthesia may provide a level of safety if immediate cautery or repacking were necessary. This would also provide the opportunity to perform an examination under anesthesia, cystoscopy, and/or proctoscopy if indicated. Suturing bleeding points in a cervical cancer is rarely successful because the suture will tear through the tumor.
A short course of pelvic radiation therapy will often bring control of the bleeding within 24 to 72 hours. There are several fractionation schemes ranging from one to ten fractions, although one has not been shown to be more effective. Alternatively, if the patient has an operable lesion, surgery should be performed once the patient is stabilized. Importantly, if the patient has received more than 4 units of packed red blood cells (pRBCs), it is prudent to assess coagulation factors (prothrombin time, partial thromboplastin time [PTT], fibrinogen, and platelet count) because the patient may have developed a “dilutional” coagulopathy and require fresh-frozen plasma (FFP), cryoprecipitate, or platelet transfusion preoperatively.
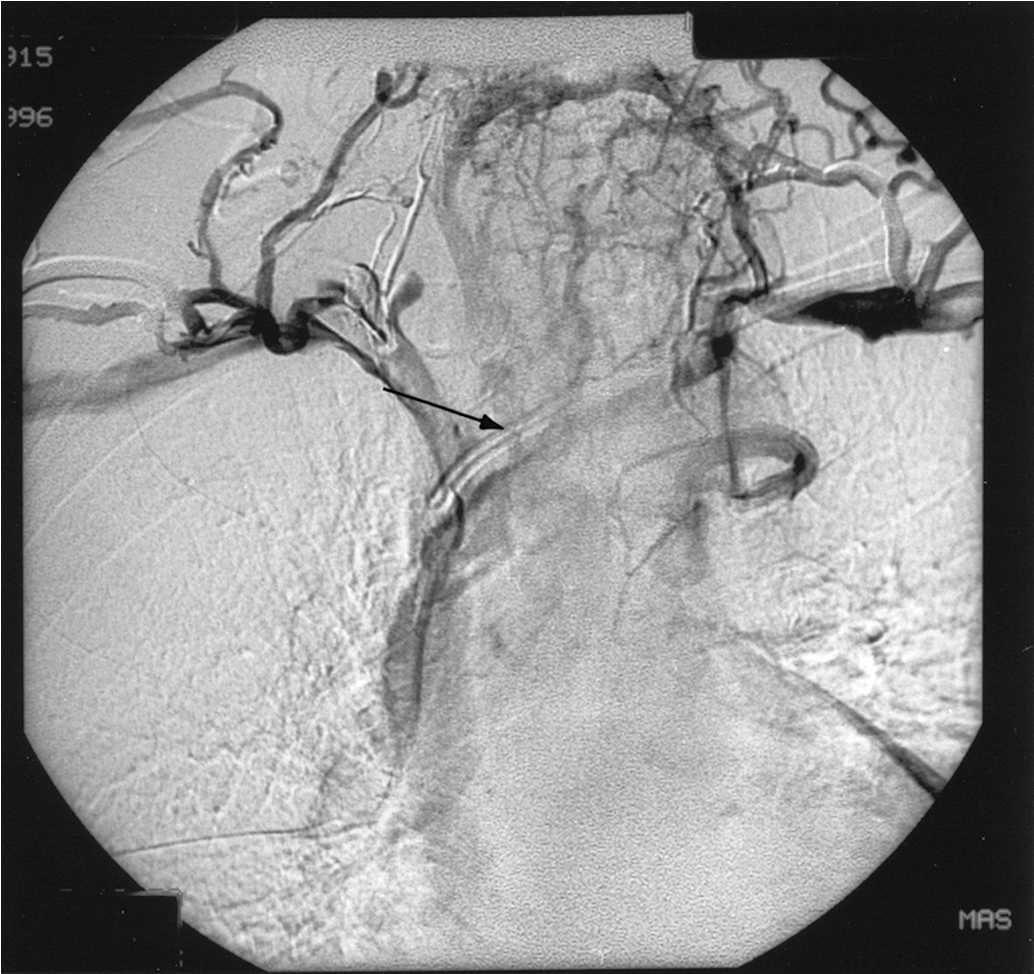
If bleeding cannot be controlled with packing or palliative radiation, or is profuse, other measures must be considered. Consultation with an interventional radiologist should be obtained to consider arteriographic embolization of the hypogastric or uterine arteries. Arteriographic evaluation will usually identify the specific bleeding vessel, and selective embolization can be accomplished. Arterial access is usually obtained through the femoral artery, and the catheter is advanced to the aortic bifurcation. Using contrast injected into the artery, the arterial vascular supply of both sides of the pelvis can be investigated to identify the specific bleeding site. Intravascular contrast can be nephrotoxic and must be used cautiously in patients who have acute or chronic renal injury or failure, who are dehydrated, or who have diabetes. Control of the bleeding site may be accomplished by continuous vasopressin infusion, a balloon catheter, or by embolization using synthetic materials (Gelfoam) or Gianturco springs imbedded with Dacron ( Fig. 13.1 ). Embolization is usually the procedure primarily chosen because the vasopressin infusion and balloon catheters require that the artery remain cannulated for a longer duration.
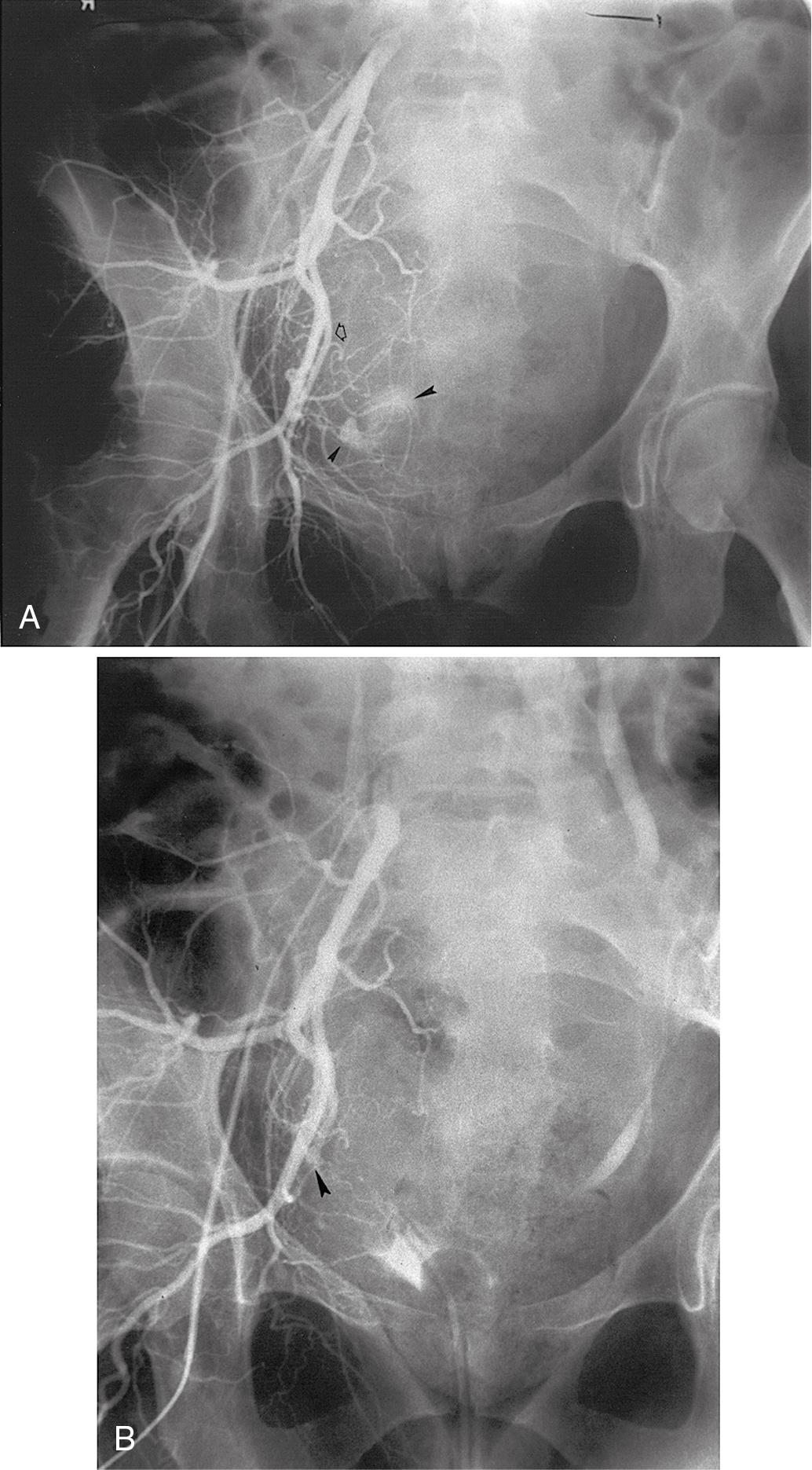
Hypogastric (internal iliac) artery ligation is usually the procedure of last resort for bleeding from a primary gynecologic cancer and is most commonly performed to control intraoperative hemorrhage; however, in situations where the cancer involves the pelvic sidewall, access to the internal iliac artery may be obstructed. Details of hypogastric artery ligation are discussed later in this chapter.
Urinary tract complications
Ureteral obstruction
Ureteral obstruction may be the primary presenting symptom of a locally advanced cervical cancer and less commonly other gynecologic cancers, including endometrial and ovarian cancers. Unilateral ureteral obstruction is often asymptomatic although flank pain may be present, especially if there is a coexistent urinary tract infection (UTI). Bilateral ureteral obstruction often presents as oliguria or anuria, an elevated serum creatinine, and other serum electrolyte abnormalities (especially hyperkalemia) . Of course, acute renal failure may arise from a number of causes, which should be investigated ( Table 13.1 ). The ureters may be obstructed as a result of local extension of the cancer, by metastases to retroperitoneal lymph nodes, or by extrinsic compression of the ureters by large masses. Uremia secondary to bilateral ureteral obstruction warrants immediate recognition and treatment. Given evidence of an elevated creatinine, evaluation of the ureters should avoid the use of nephrotoxic intravenous (IV) contrast dyes. Therefore, a renal ultrasound to assess for hydronephrosis or hydroureter is the most common initial imaging method. A non-contrasted computed tomography (CT) scan of the abdomen and pelvis can demonstrate suspected hydronephrosis and provides the additional benefit of evaluating disease burden. A Lasix renogram (renal scan with Lasix) may be helpful to access if the kidney remains viable. If bilateral ureteral obstruction is diagnosed, the patient should be rapidly evaluated to determine the true extent of the cancer before any intervention is undertaken. If the cancer appears to be locally advanced and not widely metastatic, relief of the ureteral obstruction should be attempted by cystoscopy and placement of retrograde ureteral stents. If stent placement is unsuccessful, then percutaneous nephrostomy (PCN) tubes should be inserted. Dialysis may be necessary in extreme circumstances until the obstruction can be relieved. Post-obstructive diuresis and correction of serum electrolytes must be carefully and closely evaluated and managed in the several days after relief of the ureteral obstruction.
| Disorder | Example |
|---|---|
| Prerenal Failure | |
| Hypovolemia | Skin, gastrointestinal, or renal volume loss; hemorrhage, sequestration of extracellular fluid (pancreatitis, peritonitis) |
| Cardiovascular failure | Impaired cardiac output (infarction, tamponade); vascular pooling (anaphylaxis, sepsis, drugs) |
| Postrenal Failure | |
| Extrarenal obstruction | Urethral occlusion; bladder, pelvic, or retroperitoneal neoplasms; surgical accident; calculi |
| Intrarenal obstruction | Crystals (uric acid, oxalic acid, sulfonamides, methotrexate) |
| Bladder rupture | Trauma |
| Acute Tubular Necrosis | |
| Postischemic | All conditions listed above for prerenal failure |
| Pigment induced | Hemolysis (transfusion reaction); rhabdomyolysis (trauma, coma, heatstroke, severe exercise, potassium or phosphate depletion) |
| Toxin induced | Antibiotics; intravenous contrast material; anesthetic agents; heavy metals; organic solvents |
| Pregnancy related | Septic abortion; uterine hemorrhage; eclampsia |
Complications of PCN placement include a high frequency of UTIs and pyelonephritis (70%), catheter occlusion (65%), and bleeding (28%). Seventy percent of patients will have recovery of renal function after PCN placement.
Comment needs to be made of circumstances in which the physician, patient, and family must seriously consider the possibility that relief of the obstruction may not be in the patient’s best interest. These clinical situations include the following:
- •
A patient who presents with a widely metastatic malignancy for which there is little significant opportunity to provide effective therapy.
- •
A patient who has previously been treated for cervical cancer with radiation therapy and has bilateral obstruction secondary to recurrent pelvic disease. Would the patient wish to have renal function restored in order to receive palliative chemo/immune therapy that may not improve quality or duration of life?
- •
Finally, careful evaluation should be made to be certain that the obstruction is not a result of retroperitoneal fibrosis caused by prior radiation therapy for which urinary diversion would be most appropriate.
Often, patients with bilateral ureteral obstruction are uremic and comatose. Decisions regarding intervention and care then fall to the next of kin, who must make the difficult decisions regarding intervention that may reverse the uremia but lead to death from other complications of the cancer or palliative treatments versus allowing the patient to expire peacefully in a uremic coma. Compassionate and knowledgeable consultation and advice with an experienced gynecologic oncologist are crucial in these difficult circumstances.
Unilateral ureteral obstruction at the time of initial presentation may not require stent or PCN placement if the patient’s renal function is normal and therapy (e.g., pelvic radiation therapy) is expected to control the cancer and relieve the obstruction. Placement of a PCN or stent in these circumstances must be balanced against the potential complications that might delay or interrupt therapy ( Fig. 13.2 ). A Lasix renogram can aid in evaluating the function of the obstructed kidney. If the organ is still viable, placement of a stent to avoid loss of function while awaiting tumor shrinkage from therapy can be helpful.
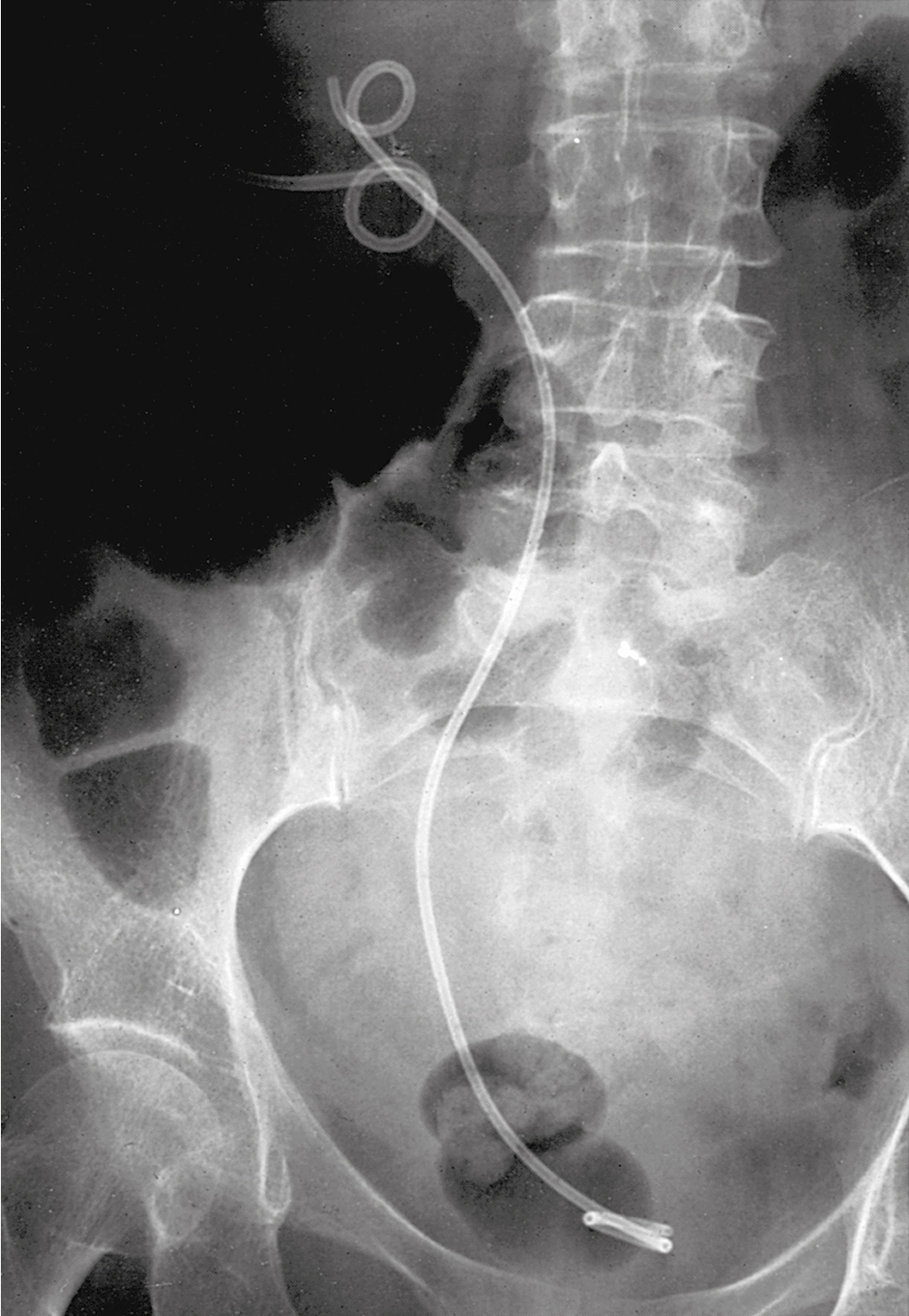
Urinary outlet obstruction (obstruction of the urethra) by a cancer that has invaded the anterior vaginal wall (vaginal, vulvar, or cervical cancers) is usually managed by placement of a Foley catheter. If a Foley catheter cannot be placed, either a suprapubic catheter or PCNs should be considered.
Urinary tract fistulas
Vesicovaginal fistula caused by a primary gynecologic cancer is relatively rare and is more commonly caused by surgical injury or radiation therapy (discussed later). Nonetheless, some patients will present with cancer that has eroded into the bladder, with subsequent loss of integrity between the bladder and vagina resulting in urinary leakage. Urinary leakage is nearly always constant (as opposed to stress or urge incontinence, which are intermittent). Overflow incontinence may result in continuous urine leakage but is usually distinguished by further evaluation. The diagnosis is most often made by speculum examination and visualization of the fistula and continuous urine loss. A smaller fistula may require instillation of dye diluted in saline (methylene blue, indigo carmine, infant formula) into the bladder in order to identify leakage into the vagina. Ureterovaginal fistula due to pelvic cancers is rare. Imaging to diagnose a small fistula using CT with IV contrast or an intravenous pyelogram (IVP) may be helpful. On occasion, cystoscopic retrograde injection of contrast dye into the distal ureter may be necessary.
Correction of the fistula caused by a cancer cannot be considered until the cancer has been eradicated. In the interim, while cancer therapy is undertaken, the patient may be very uncomfortable from the continued loss of urine. Attempts to diminish the leakage should be undertaken. Placement of a Foley catheter will often partially divert urine from the fistula into the catheter. A fitted diaphragm with foley catheter in the center, to collect urine, has been used with success. PCN tubes may also reduce the volume of urine that reaches the bladder. Good perineal hygiene and barrier ointments are helpful to reduce vulvar skin irritation. Urinary diversion (urinary conduit) may be the only complete solution to profuse vaginal urinary leakage; however, timing of this major surgery should be weighed against the delay in primary cancer therapy.
Gastrointestinal complications
Gastrointestinal obstruction
Intestinal obstruction as a presenting symptom of a gynecologic cancer is most commonly caused by advanced ovarian cancer. In cases of small bowel obstruction, initial therapy should include correction of fluid volume and electrolytes, nutritional assessment, and bowel rest. If nausea and emesis continue despite antiemetics, a nasogastric (NG) tube should be placed for decompression. Alternatively, an NG tube may be placed immediately if there is significant stomach distention requiring decompression. Assessment of intestinal patency by a CT scan with oral contrast should be performed to have a better understanding of the location and extent of obstruction. The colon should also be evaluated to exclude the possibility of colonic obstruction, which would need to be relieved at the same surgical procedure. In most cases of ovarian cancer, surgical exploration is necessary to establish a diagnosis, stage the cancer, debulk the tumor, and relieve the obstruction. Although patients presenting with gastrointestinal (GI) obstruction may be severely malnourished, the role of preoperative total parental nutrition (TPN) is debatable. The highest quality evidence suggests that at least 10 days of TPN administration is necessary to provide a benefit of reduced postoperative complications.
On occasion, the preoperative assessment (usually with a CT scan) discovers very advanced disease (extensive carcinomatosis), which would be unlikely to be successfully debulked. In these patients, neoadjuvant chemotherapy, rather than surgical intervention, may be the best initial treatment option . If this therapeutic strategy is taken, GI decompression (NG tube or gastrostomy tube) and parenteral nutritional support (TPN) will be required for several weeks while the neoadjuvant chemotherapy has the opportunity to result in tumor response and relief of the intestinal obstruction. Fortunately, many patients with ovarian cancer will regain intestinal function after two or three cycles of chemotherapy.
Short bowel syndrome may result from extensive resection of the small bowel, colon, or both. The syndrome is characterized by frequent diarrhea, fluid and electrolyte depletion, malabsorption, and weight loss. Depending on the extent and location of the intestinal segment(s) resected, malabsorption of nutrients may include copper, zinc, chromium, selenium, essential fatty acids, vitamins A and E, biotin, thiamine, and vitamin B 12 . Over time, the remaining small bowel often adapts, and fluid and nutrient absorption is improved. In the interim, attempts to relieve short bowel syndrome should be directed at decreasing transit time by the use of an “elemental” diet and Imodium or Lomotil, cholestyramine (to decrease irritation of bile salts on the colonic mucosa), and somatostatin (to decrease intestinal digestive fluid production). In extreme cases, support with IV fluids and TPN may be necessary for up to several months.
In most cases, colonic obstruction should be immediately corrected to prevent colonic perforation, peritonitis, sepsis, and death. Most often, the colonic obstruction is resolved by surgical intervention (colostomy, partial colectomy with anastomosis, ileostomy). In cases where colostomy is performed, reversal (“take down”) of the colostomy should be considered if the patient has a good response to chemotherapy. Other management options include placement of a cecostomy tube or placement of a self-expanding metal intraluminal stent. In a series of gynecologic oncology patients, rates of technical success of stent placement ranged from 75% to 88%; however, clinical success was lower, with more than one-third (33% to 38%) of patients with stent placements requiring further intervention for either stent complications or failure. Stents should not be placed in setting of multi-organ dysfunction. Due to increased risk of perforation, stents are contraindicated when bevacizumab will be used for treatment of ovarian cancer. Advantages of stents include the avoidance of major surgery and a possible stoma.
Intestinal obstruction often occurs late in the course of progressive ovarian cancer. In these situations, superb clinical judgment is required to obtain an optimal palliative outcome because not all patients with recurrent ovarian cancer and intestinal obstruction benefit from surgical intervention. It seems intuitive that patients with colonic obstruction should be offered surgery to create a colostomy, ileostomy, or cecostomy. A patient with a small bowel obstruction requires careful thought and triage. Initially, conservative management with IV fluid and electrolyte replacement and NG tube decompression should be instituted. Some patients may reestablish bowel function with a few days of “bowel rest.” If the obstruction persists, the decision to surgically relieve the intestinal obstruction must be made with careful consideration as to expected outcomes. Of course, a patient who has a small bowel obstruction caused by adhesions should undergo surgery. The challenge in decision making comes when it is clear that the patient has recurrent ovarian cancer. Many investigators have attempted to identify factors that would predict successful outcomes such as surviving 30 days, achieving discharge from the hospital, ability to take oral fluids, postoperative complications, and death. Information regarding risk factors contributing to poor surgical outcomes are based on retrospective series, which have inherent selection bias. In general, clinical factors resulting in poor outcomes seem to include: evidence of carcinomatosis, presence of ascites, complete (versus partial) small bowel obstruction, and poor nutritional status. Factors which are associated with better surgical outcomes include: availability of effective subsequent therapy, prior use of radiation therapy, length of time since prior therapy, and potential for the ovarian cancer to be “platinum sensitive.”
If surgical intervention is deemed appropriate, surgical procedures might include bypass of involved segments of small bowel (entero-enterostomy), bowel resection with anastomosis, or ileostomy. Unfortunately, in every surgeon’s experience, there are patients who undergo laparotomy only to find such extensive carcinomatosis that they are deemed inoperable. The decision to operate, then, should be based on a clear communication between the surgeon and patient regarding expectations and definitions of “success.” In the authors’ experience, which is reasonably representative of the general literature, the median survival time after small bowel obstruction surgery was 88 days, and only 14% of patients were alive at 12 months. In addition, 49% of patients had at least one significant postoperative complication, including wound infections, enterocutaneous fistula, sepsis, and recurrent obstruction.
If the decision is made not to operate, further management is also complex, including methods to palliate vomiting (percutaneous gastrostomy rather than continued drainage with an NG tube is recommended) and whether continuation of IV fluids or TPN should be offered in a hospice setting.
Gastrointestinal fistulas
Rectovaginal fistula may be discovered at the time of primary diagnosis of cervical, vaginal, or vulvar cancers. Involuntary loss of feces, flatus, and mucous discharge are the most common symptoms. If the patient has vulvar pain and excoriations, a fistula from the small intestine should be suspected. In this instance, a fistulogram can be helpful if definitive diagnosis of the source of a fistula is in question, and a CT scan with oral contrast should be performed to define the exact anatomic structures involved. If a rectovaginal fistula is found, diversion with a loop colostomy or end-colostomy is suggested to divert the fecal stream and allow prompt treatment of the cancer (usually radiation therapy). If vulvar cancer is so advanced as to cause a rectovaginal fistula, some surgeons manage the cancer and the fistula in the same surgical procedure (e.g., a posterior pelvic exenteration, colostomy, and modified radical vulvectomy). Others have had excellent results treating locally advanced vulvar cancer with radiation therapy and concurrent radiosensitizing chemotherapy, thereby preserving the rectal sphincter. Colostomy diversion is still suggested for patient comfort and hygiene. If the cancer treatment is successful, attempts to close the fistula are reasonable, and if successful, the colostomy may ultimately be reversed.
Enterovaginal fistulas rarely complicate the initial cancer diagnosis and more often occur as a result of complications of therapy (radiation) or at the time of cancer recurrence. The flow of intestinal contents out of the vagina is usually liquid and caustic to vulvar skin. Thorough evaluation of the upper and lower GI tracts and the urinary tract is mandatory because many of these fistulae are “complex,” involving more than one viscous and more than one defect. Surgical intervention is necessary in most cases to either resect or isolate the involved bowel. If resection is not possible, the fistulized bowel will need to be isolated and excluded from the intestinal stream. Because the isolated bowel will continue to create succus entericus and subsequent continued vaginal drainage, resection is generally preferred.
Biliary obstruction
Obstruction of the biliary tree by gynecologic cancers is rare and is usually associated with far advanced cancers and limited life expectancy. Nonetheless, the resulting jaundice and pruritus caused by the obstruction is distressing to the patient and her family. Surgical relief of the obstruction is usually impossible because of the extent of cancer involvement in the region; however, endoscopic placement of a stent in the common bile duct often will resolve the symptoms and provide a better quality of life. If a stent cannot be placed as a result of extreme compression or other technical reasons, percutaneous placement of a drainage tube into the dilated biliary tree will also resolve symptoms.
Metastatic adenopathy high in the para-aortic chain resulting in biliary obstruction can obstruct the duodenum, leading to gastric outlet obstruction. Although surgical intervention (gastrojejunostomy) or self-expanding metal stents may correct the anatomic problem, careful consideration should be given to the patient’s life expectancy prior to pursuing intervention. These are similar considerations to those made in women with small intestinal obstructions and recurrent ovarian cancer discussed previously. In women with just days or weeks to live, placement of a gastrostomy tube may be more prudent.
Treatment-related complications
Surgical
Intraoperative and postoperative hemorrhage
Intraoperative management of vascular complications.
Surgery for gynecologic cancer often requires extensive dissection in the retroperitoneal space, which may be distorted by cancer metastatic to lymph nodes or invading adjacent structures. It is not surprising, then, that injury to pelvic veins and arteries is common and may result in significant intraoperative blood loss and hemorrhage. Surgeons must be prepared for this and have in their armamentarium the tools and skills to bring a stop to the bleeding.
Before attempting to bring final control to a significant bleeding area, a few basic principles should be used. First, the patient’s blood volume and coagulation factors must be maintained at all times. Poor communication between the surgeon and the anesthesiologist can lead to significant hypovolemia and cardiovascular instability if resuscitation does not match losses. Frequent serial laboratory assessments are critical in this setting. Loss of coagulation factors during intraoperative hemorrhage results in continued bleeding that cannot be controlled by surgical means . Should this occur, the surgeon should pack the involved area and delay further attempts to stop hemorrhage to allow replacement of blood volume (pRBCs), coagulation factors (FFP, platelets, and cryoprecipitate), and acquisition of appropriate assistance. When the patient is stable and the team is fully prepared, the packed area should be exposed a small area at a time to identify the specific bleeding site.
Before attempting to control the cause of bleeding, the adjacent anatomy should be identified and protected. In particular, the ureter, adjacent vessels, and viscera must be recognized to avoid further injury. In most cases of arterial bleeding, the artery can be isolated and controlled. Small arteries may be best controlled by vascular clips, but larger arteries may require suture ligation with 4-0 or 5-0 vascular suture (Prolene). This holds for injury to the aorta and common and external iliac arteries. Injury to the internal iliac (hypogastric) artery may be controlled with total ligation of the artery. Patency of distal arteries should be confirmed throughout the remainder of the procedure and postoperatively. In rare instances, arterial injuries must be managed by vascular grafting.
Venous bleeding in the pelvis is a more common problem given the fragility of the thin vein walls and the extensive network of pelvic venous plexus. Often a specific bleeding point cannot be identified but, after several minutes of direct pressure on the bleeding area, a clot will form over the low-pressure veins, and the bleeding will resolve. If it does not, control will have to be achieved with vascular clips, clamps, and suture ligature. Slow but persistent oozing from unidentified vessels can often be controlled by products that either serve as a matrix for clotting (Avitene, Surgicel, Gelfoam) or supply clotting factors that complete the clotting cascade (CoSeal, TISSEEL, FLOSEAL). After application of any of these products, pressure should be applied to the site for 5 minutes and then the site should be reevaluated. If bleeding has not been controlled, it may be necessary to place a pack and transfer the patient to an intensive care unit (ICU). In all cases, it should be emphasized that prompt replacement of deficient clotting factors by transfusion of FFP, cryoprecipitate, and platelets is critical to achieve hemostasis in the face of hemorrhage .
Replacement of platelets and clotting factors in patients with massive transfusion and microvascular bleeding is dependent on clinical and surgical assessments. Guidelines have been provided by the American Society of Anesthesiologists task force regarding replacement of these products. In general, platelet transfusion is rarely indicated for platelet counts greater than 100,000/μL and usually indicated for counts less than 50,000/μL (with intermediate platelet counts 50,000/μL to 100,000/μL, transfusion should be based on the risk of bleeding). FFP therapy is indicated in massively transfused patients with microvascular bleeding or hemorrhage if the PT or aPTT values exceed 1.5 times the normal values. In cases of massive intraoperative blood loss, it may be prudent to administer FFP empirically. This strategy is aimed at preventing the patient from becoming hypocoagulable while awaiting the laboratory results (PT and PTT), which may take nearly an hour and another hour to thaw the FFP before it is available to be administered. Cryoprecipitate transfusions are recommended for correction of microvascular bleeding in massively transfused patients with fibrinogen concentrations less than 80 to 100 mg/dL. For trauma patients with massive blood loss, on the order of 30% to 40% of their blood volume, a transfusion ratio of 1:1:1 for pRBCs, FFP, and platelets has been consistently reported to be associated with decrease in death caused by hemorrhage. If surgical bleeding has taken on this level of severity, such a massive transfusion protocol should be initiated .
Bleeding from the sacrum is usually not encountered except in the course of performing a total pelvic exenteration or rectosigmoid resection or during a sacral-colpopexy. If bleeding from the sacrum cannot be controlled by suture ligatures or vascular clips, placement of sterile thumbtacks, pushed into the sacral bone, will usually compress veins exiting the sacrum and achieve hemostasis .
Arterial and venous bleeding may both be encountered in minimally invasive surgery as well as open surgery. The same principles discussed earlier hold true in minimally invasive surgery. The intra-abdominal pressure from the pneumoperitoneum serves as a tamponade of sorts, often giving the surgeon the time to assess what is bleeding and decide on a method for achieving hemostasis without significant blood loss.
Hypogastric (internal iliac) artery ligation.
When the usual steps to control hemorrhage have failed, hypogastric artery ligation should be performed. Although the arterial blood supply to the pelvis is rich with anastomosis, ligation of the hypogastric arteries will usually decrease the arterial and venous pressure to the point at which (in a patient who is not hypocoagulable) venous bleeding will slow and can either be controlled by ligature or will clot with prolonged packing.
To safely perform hypogastric artery ligation, the vascular anatomy must be exposed, and adjacent structures, especially the ureter, must be identified. A retroperitoneal approach should be taken. The peritoneum overlying the psoas muscle (lateral to the external iliac artery) should be incised parallel to the artery. As the peritoneum is mobilized medially, the external iliac artery will first be identified. Dissection cephalad along the external iliac artery will identify the common iliac artery and the bifurcation of the internal iliac artery. Invariably, the ureter crosses the pelvic brim at the bifurcation of the common iliac artery. At this location, the ureter will be identified attached to the medial peritoneum. Further opening of the retroperitoneal space keeping the iliac vessels lateral and the ureter medial will create the pararectal space and further expose the internal iliac artery. Be aware that the common external and internal iliac veins lie just beneath their respective arteries. Without clear identification of these veins, injury can occur, which will further complicate the procedure. The hypogastric artery bifurcates into an anterior and posterior branch 2 to 3 cm from its origin from the common iliac artery. Because most bleeding arises from the blood supply from the anterior division, the anterior branch only should be ligated if at all possible. Ligation of the posterior branch increases the risk of buttock pain and potential necrosis of the gluteus. A right-angle clamp should be carefully passed from lateral to medial beneath the hypogastric artery. A heavy suture (e.g., 0-silk) should be used to ligate the artery ( Fig. 13.3 ). There is no reason to transect the artery between two ligatures. It is usually best to perform bilateral hypogastric artery ligation as the collateral blood supply crosses over the midline.
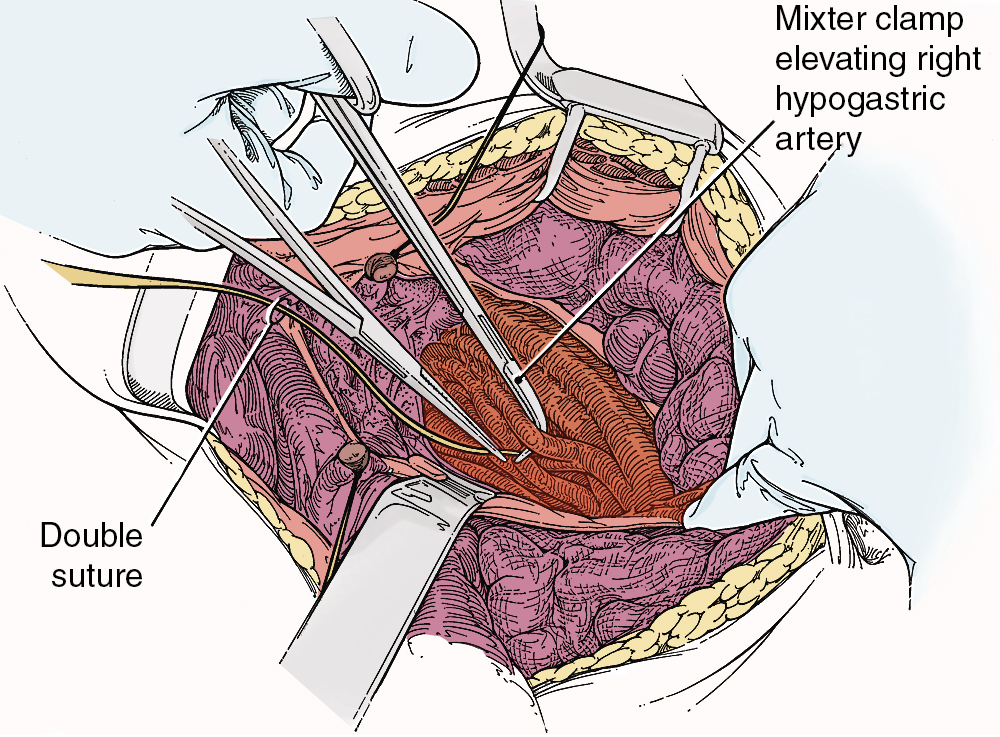
Finally, if all methods to control hemorrhage have been unsuccessful, the bleeding site should be packed firmly, and the abdomen closed temporarily. The patient should be taken to the surgical ICU and stabilized. Central monitoring and blood product replacement should be the primary focus of management. After the patient is stabilized, attempts at angiographic embolization should be considered. Ultimately, after 24 to 48 hours, the patient should be returned to the operating room, reexplored, and the pack removed carefully. Thankfully, many times the bleeding will have stopped as a result of compression of the injured veins and correction of the hypocoagulable state.
Intraoperative and postoperative genitourinary tract injuries
Intraoperative genitourinary injuries.
Given the close anatomic relationships of the gynecologic organs and the urinary tract, ureteral and bladder injury are to be anticipated, even in the hands of the most skilled surgeon. Prevention of urinary tract injury is predicated on the identification of the key urinary tract structures before embarking on radical or extended surgery for gynecologic cancers. Retroperitoneal exploration by opening the lateral retroperitoneal spaces (pararectal and paravesical spaces) allows for identification of the ureter and lateral bladder as well as key vascular structures. If the medial pelvic peritoneum requires resection, the ureter must be detached from the peritoneum and mobilized laterally (ureterolysis). The ureter may be dissected throughout its entire pelvic course to the bladder, although between the uterine artery crossing the ureter and the insertion of the ureter into the bladder, techniques similar to those required for a type II or III radical hysterectomy will need to be used. Identification of the bladder is usually not a problem; however, because of anatomic distortion by advanced cancer, radiation therapy, or extensive adhesions from prior surgery, the bladder sometimes is not easily recognized. One simple method to identify the bladder is to fill it retrograde through the indwelling Foley catheter. With the bladder distended, dissection of the uterus or tumor from the bladder may be facilitated. Opening the retropubic space (space of Retzius) and the paravesical space also facilitates identification and protection of the bladder.
Injury to the bladder is usually easily corrected at the time of surgery. Incisions in the dome of the bladder should be closed in two layers with 3-0 or 2-0 delayed absorbable suture. The bladder should be allowed to heal by leaving the Foley catheter to dependent drainage for approximately 5 to 7 days. Cystotomies at the base of the bladder have a higher risk of fistula formation and ureteral occlusion. Furthermore, they may be more difficult to recognize. Whenever there is concern about potential bladder injury, the bladder should be filled in a retrograde fashion with either sterile infant formula or saline dyed with methylene blue or indigo carmine. The authors prefer infant formula because the formula does not stain tissues, which might obscure the site of injury. After the cystotomy in the base of the bladder is identified, it is important to assess the location of the ureteral orifices. This may be accomplished by cystoscopy or by opening the dome of the bladder and directly visualizing the orifices. The administration of IV indigo carmine or methylene blue may aid in the identification of the orifices. Closure of the cystotomy should again be accomplished using two layers of delayed absorbable suture. If the cystotomy is near the ureteral orifice, retrograde placement of a ureteral stent is prudent to ensure that there is no occlusion or narrowing of the ureter or ureteral orifice. If the pelvis has been previously radiated, the authors recommend placement of an omental J-flap between the bladder and the vaginal cuff to bring a new, nonirradiated blood supply into this area. The Foley catheter should be left to drain for several weeks, and a cystogram should be obtained before the decision to remove the catheter.
Injury to the ureter as it passes through the pelvis may be managed by several methods depending on the location and extent of the injury. Ureteral injury above the pelvic brim is usually best managed by end-to-end anastomosis over a ureteral stent ( Fig. 13.4 ). Injury below the pelvic brim may be best corrected by a ureteroneocystostomy with psoas hitch or a Boari flap ( Fig. 13.5 ). In either method of repair, placement of a closed suction drain in the pelvis is advised to prevent a urinoma from a leak of the anastomosis.
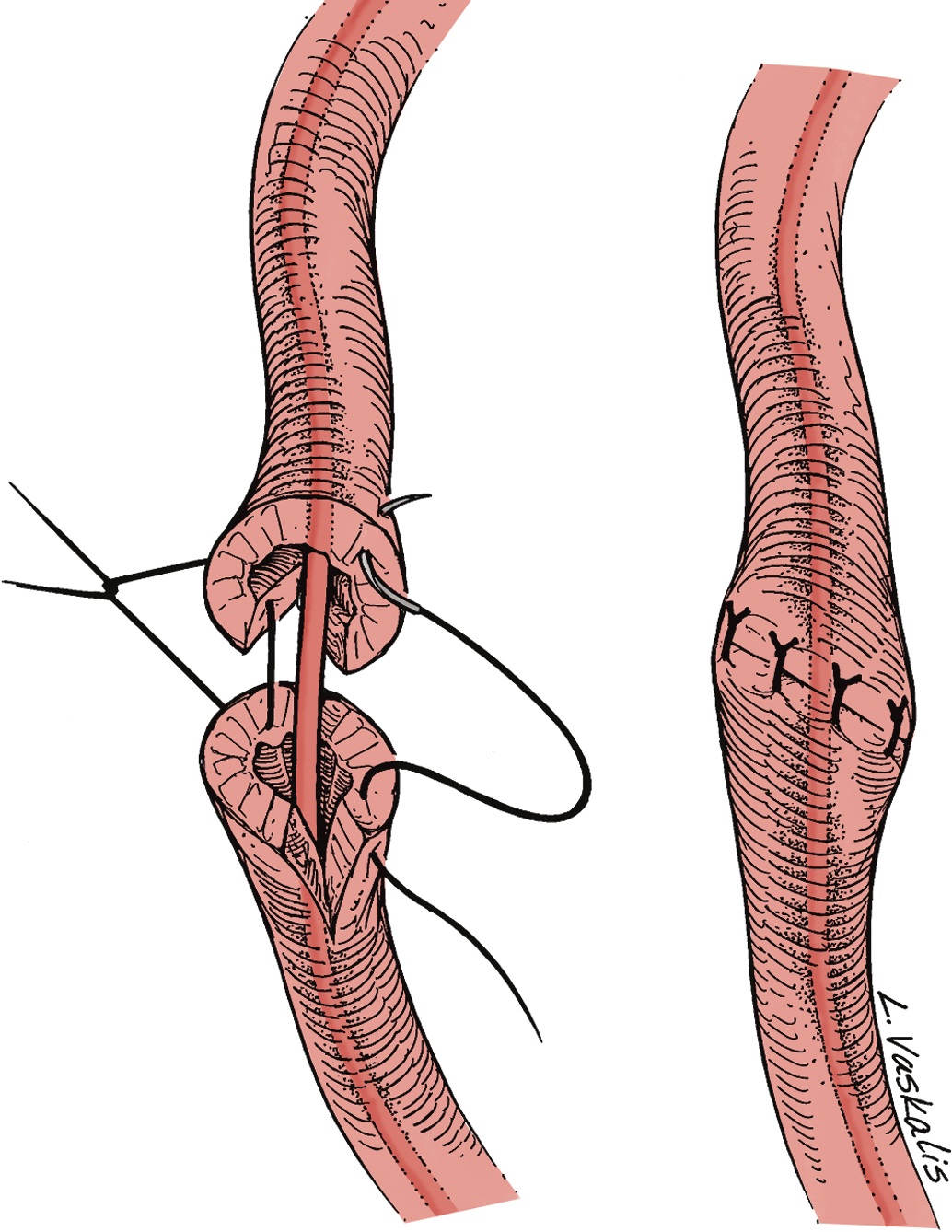
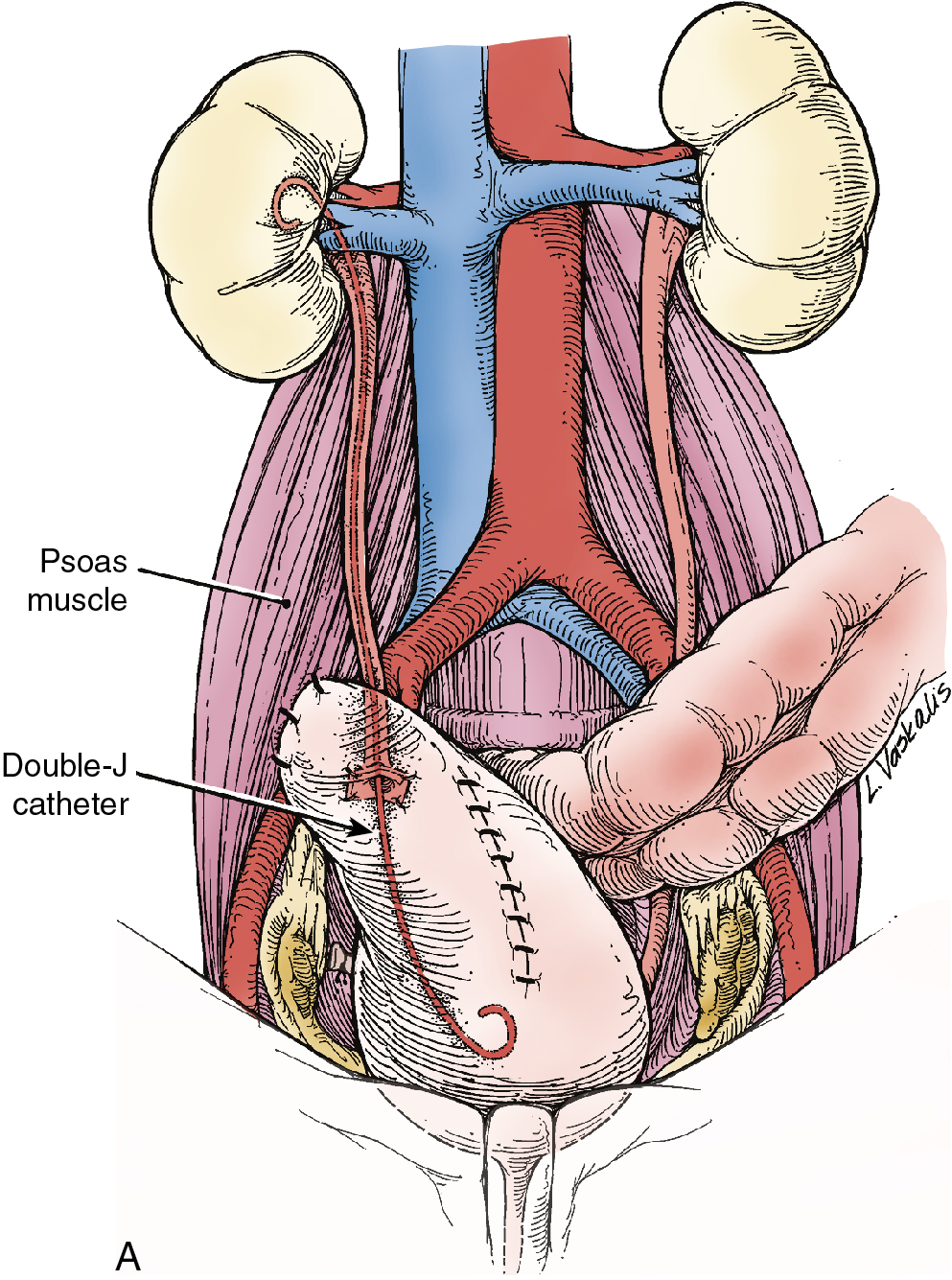
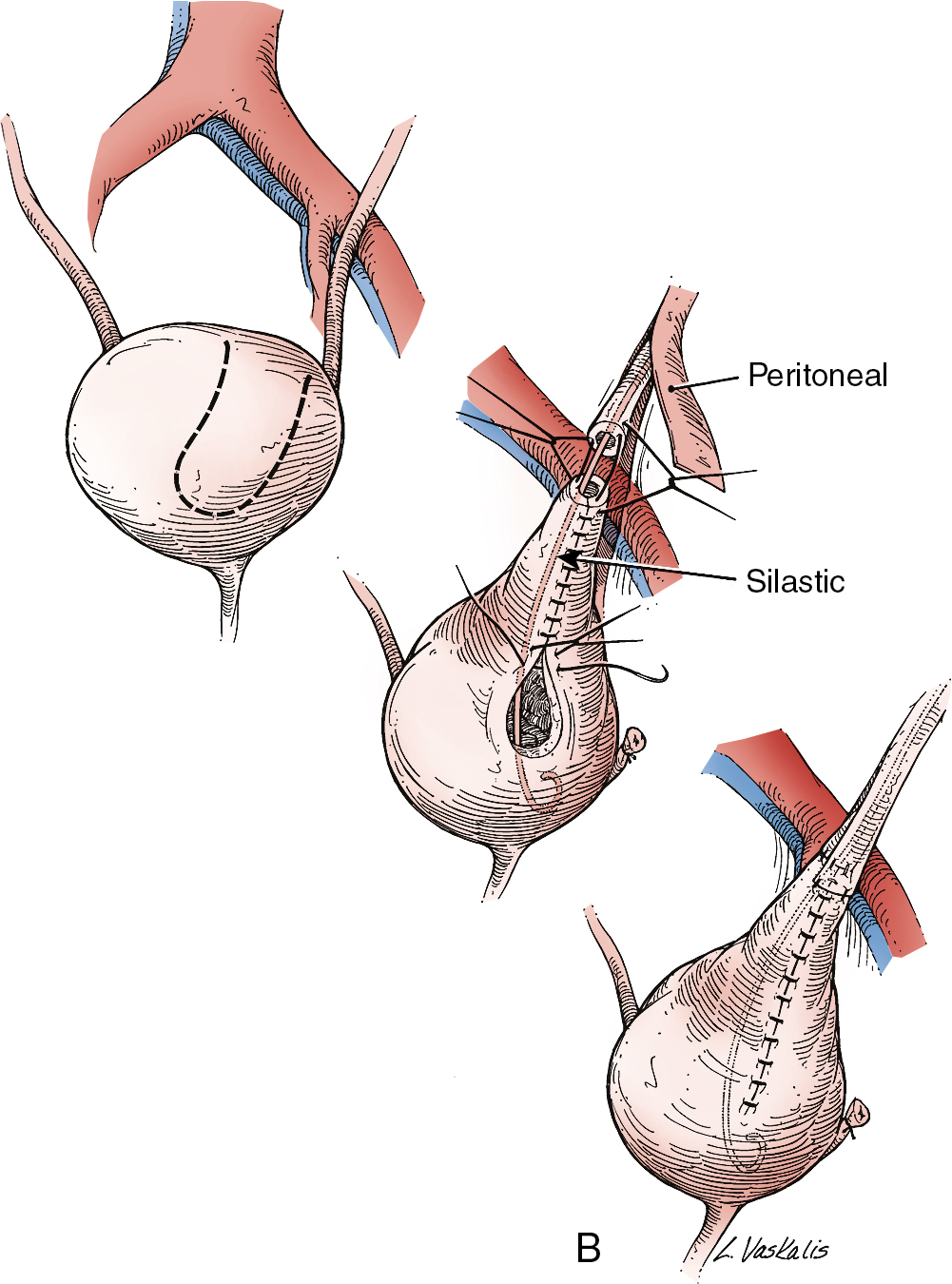
In cases of suspected injury to the ureter, IV indigo carmine or methylene blue should be injected, and the pelvis should be inspected for spill of dye-colored urine. If the extent of ureteral injury is a clamp crush or ligature, placement of a ureteral double-J stent, to be left in place for 6 weeks, will usually allow the injured ureter to recover and at the same time prevent ureteral stricture.
Postoperative genitourinary injuries.
Unless bilateral ureteral obstruction has been caused by surgery, most patients with postoperative anuria or severe oliguria will have these findings secondary to prerenal hypovolemia, which will resolve with hydration and diuresis. Unilateral ureteral injury may not be recognized until several days postoperatively, manifest by flank pain, pyelonephritis, or a slight rise in serum creatinine. The volume of urinary output is rarely altered. When postoperative ureteral obstruction is suspected, evaluation may include renal ultrasound, CT scan with IV contrast (when the serum creatinine level is normal), or Lasix renogram. If ureteral obstruction is discovered, initial management should include cystoscopy with retrograde stent placement. If stent placement is successful, the obstruction is likely a result of tethering from nearby sutures or extrinsic compression from a mass (hematoma, lymphocyst, tumor). Leaving the stent in place for 6 weeks and then reevaluating with follow-up CT urogram or cystoscopy is recommended. If a stent cannot be placed, consideration should be given to reexploration to correct the obstruction. If reoperation is not reasonable, a PCN tube should be placed to preserve the renal unit’s function until definitive surgical correction can be accomplished.
Urinary tract fistulae are recognized complications of radical hysterectomy for the primary treatment of cervical cancer and other radical surgical procedures where the ureter is manipulated. This serious complication is reported to occur in approximately 1% to 3% of cases. It is thought that the extensive dissection of the distal ureter and base of the bladder may lead to ischemic necrosis and the development of a vesicovaginal or ureterovaginal fistula. The risk of fistula is increased when surgery is performed after prior pelvic radiation therapy.
Vaginal leakage of fluid during the first 10 weeks postoperatively is an ominous finding and requires evaluation for a urinary tract fistula. Confirming the presence of a fistula and, if present, identifying its location are the next priorities. Office-based examinations include retrograde filling of the bladder with dyed (e.g., methylene blue, infant formula, indigo carmine) saline and inspection of the vaginal vault for leakage (vesicovaginal fistula) and administration of IV indigo carmine or oral pyridium with vaginal inspection for ureterovaginal fistula. Both of these diagnostic methods may be falsely negative. Even if negative, in cases of continued vaginal leakage, a definitive CT scan with IV contrast (CT urogram or CT of the abdomen and pelvis with delayed-phase contrast) should be obtained. Acute vesicovaginal fistula should be managed by decompression of the bladder by placement of an indwelling Foley catheter to allow continuous drainage. This management often allows the fistula to close spontaneously. If a ureterovaginal fistula is discovered, a ureteral stent should be placed across the section of ureter that is fistulized. The stent may be placed retrograde at the time of cystoscopy or antegrade via a PCN. This usually allows the ureteral injury to heal “over” the stent. Throughout attempts at conservative management, prevention of UTI is an important priority. If after 6 weeks of conservative management the vesicovaginal or ureteral fistula has not resolved, surgical correction will be required.
Bladder dysfunction after radical surgery
Postoperative voiding dysfunction after type III radical hysterectomy, abdominal perineal resection (posterior exenteration), or rectosigmoid resection is commonly encountered and should be discussed as part of the preoperative informed consent. The exact frequency of occurrence depends on how the problem is diagnosed (patient report vs. prospective urodynamic testing) but occurs to a greater or lesser degree in nearly all patients. Symptoms may range from stress incontinence, to lack of bladder sensation, to inability to void completely (or not at all). While bladder dysfunction is occasionally encountered following extrafascial hysterectomy (type I) for benign disease, it appears that the extent of parametria resected is associated with the extent of bladder dysfunction.
Serial cystometric studies of patients undergoing radical hysterectomy have defined the natural history of bladder function in the perioperative period. There is a nearly uniform development of detrusor hypertonia characterized by low capacity, high resting tone, and high filling pressure in the immediate postoperative phase. Bladder insensitivity to filling is also present. The patient often has difficulty initiating her stream and may have overflow incontinence when the capacity is exceeded. Hypertonia generally subsides within 3 to 6 months, but other abnormalities often persist for years. The duration of bladder dysfunction is also variable, with extremes recognized as full recovery of bladder function to the rare patient who will require lifetime clean intermittent self-catheterization to achieve adequate bladder drainage. Many patients have persistent decrease in bladder sensation or prolonged urinary hesitancy.
It also seems clear that overdistention of the bladder aggravates bladder dysfunction. A variety of techniques have been proposed to avoid overdistention, including short- or long-term use of a urethral Foley catheter, suprapubic catheter, or clean intermittent self-catheterization. We have also found that some patients benefit from sacral neuromodulation. All techniques have their proponents and varying degrees of success, but none has been proven to be superior.
Intraoperative and postoperative gastrointestinal tract injuries
Intraoperative gastrointestinal injuries.
A mechanical bowel preparation with preoperative oral antibiotics (e.g., erythromycin and neomycin) should be planned for all patients undergoing major abdominal surgery for which colon surgery is anticipated. Several retrospective and prospective cohort studies of patients undergoing colorectal resection for colon cancer demonstrate decreases in surgical site infection, anastomotic leak, and ileus in patients receiving both mechanical and antibiotic preparation. In a prospective cohort study of over 1500 patients, mechanical preparation plus oral antibiotics resulted in lower infection (4.6% vs. 12.4%; P < .001) and lower rate of ileus (3.8% vs. 8.9%; P = .006) compared with mechanical preparation alone. A meta-analysis that included nearly 8500 patients undergoing colorectal surgery found that the combination of mechanical and antibiotic bowel preparation was associated with the lowest risk of a surgical site infection. Bowel preparation may also reduce the risk of intraoperative intestinal injury, and if injury does occur, spill of GI contents can be minimized. Mechanical methods to prepare the bowel include the use of cathartics, such as magnesium citrate, or the ingestion of 4 L of polyethylene glycol (GoLYTELY).
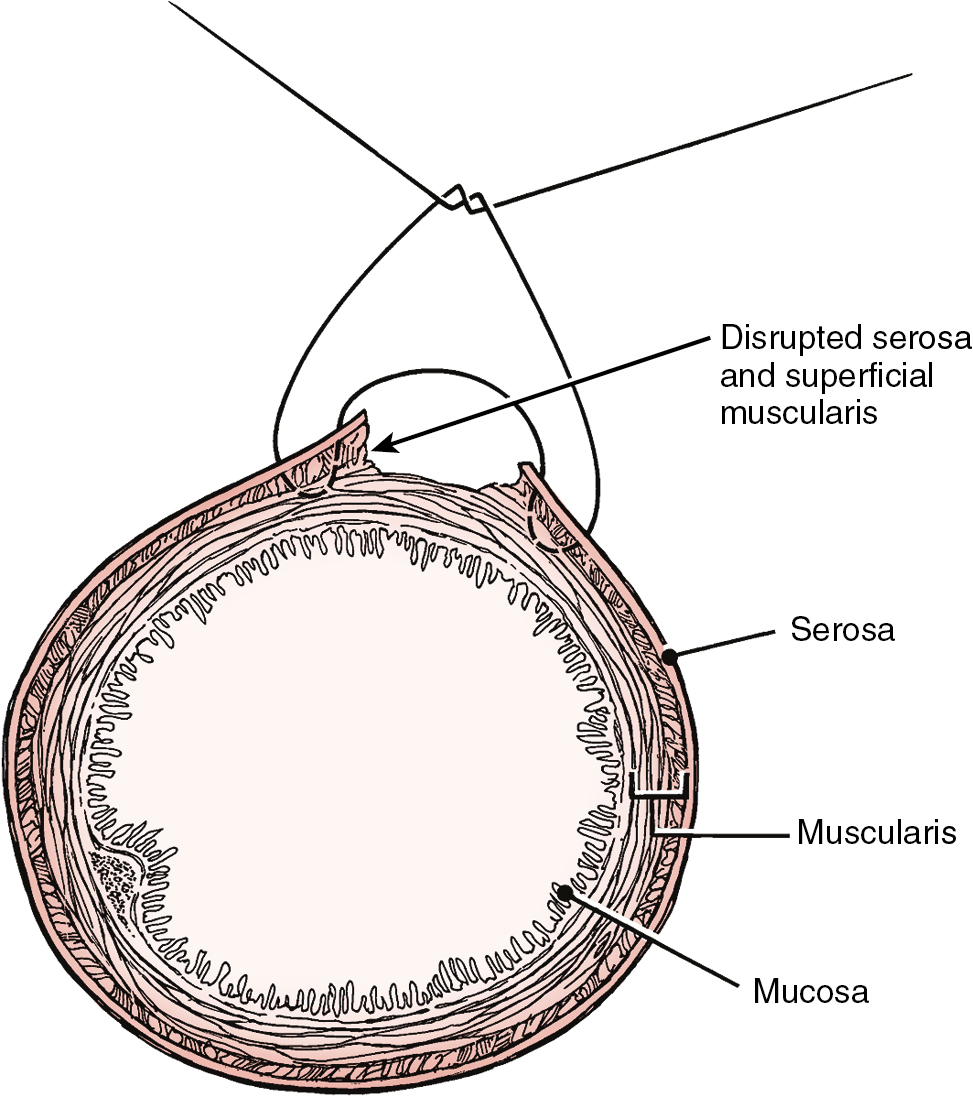
Prior abdominal surgery and radiation therapy increase the probability of intraoperative injury to the small intestine. Adhesions of the small intestine to the anterior abdominal wall or pelvic peritoneum increase the risk of entry into the intestines as adhesions are lysed. Interruption of the serosal and superficial muscular layers should be repaired with interrupted 2-0 or 3-0 sutures. Care should be taken to avoid narrowing of the intestinal caliber ( Fig. 13.6 ). If the entire thickness of the intestine is entered, the segment of bowel must be assessed to decide whether primary closure should be undertaken or whether resection and anastomosis should be performed. Primary closure is usually safely accomplished if the closure can reapproximate well-perfused bowel with no tension on the suture line and no narrowing of the bowel lumen. If these conditions cannot be met, resection and anastomosis will be necessary. Primary closure of an enterotomy should be in two layers, the first being an interrupted layer of 2-0 or 3-0 polyglycolic acid suture incorporating the mucosa and muscularis, with the knot tied into the intestinal lumen. A second imbricating layer may be of either absorbable or nonabsorbable suture and incorporates the serosa and superficial muscularis ( Fig. 13.7 ). Attention to the direction of closure of the enterotomy should ensure that the lumen is not narrowed.
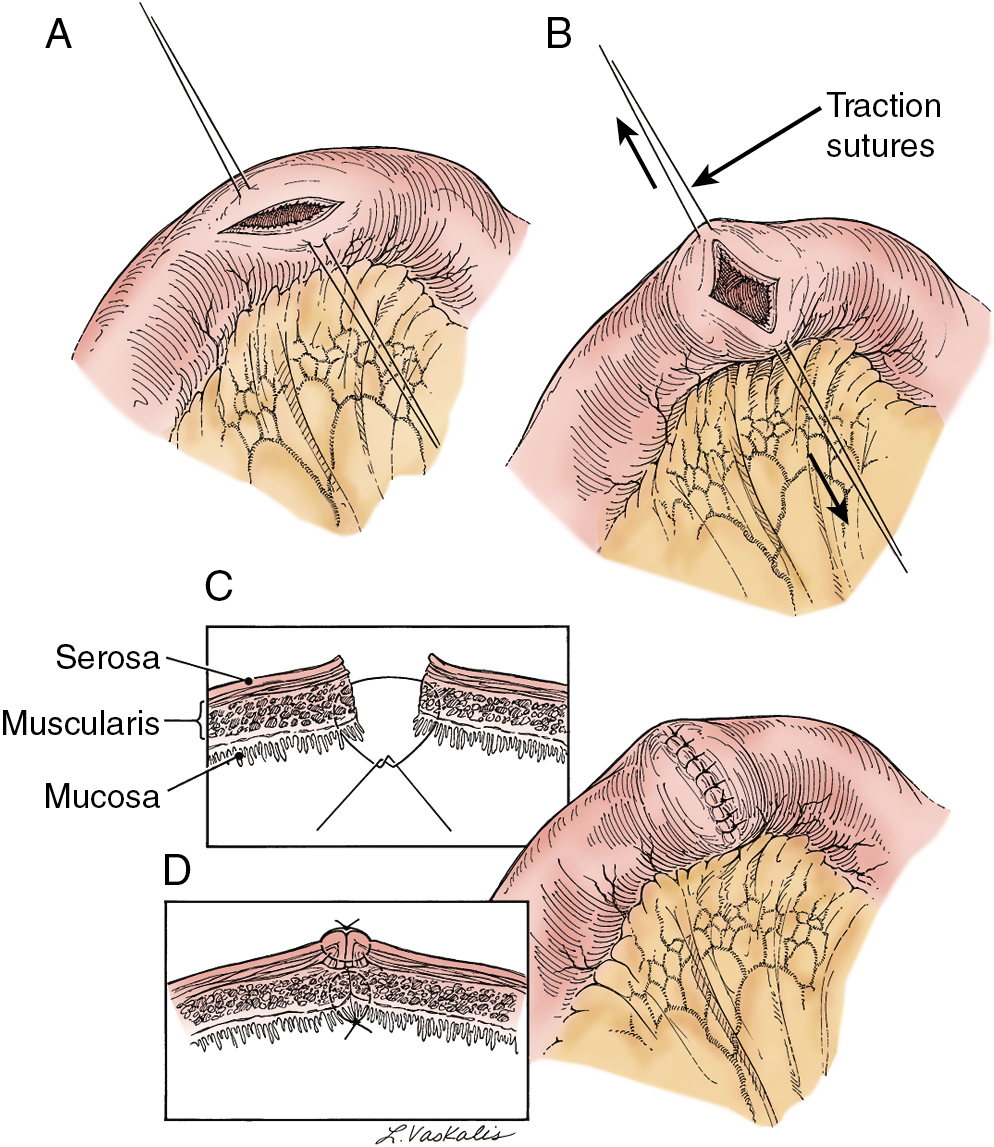
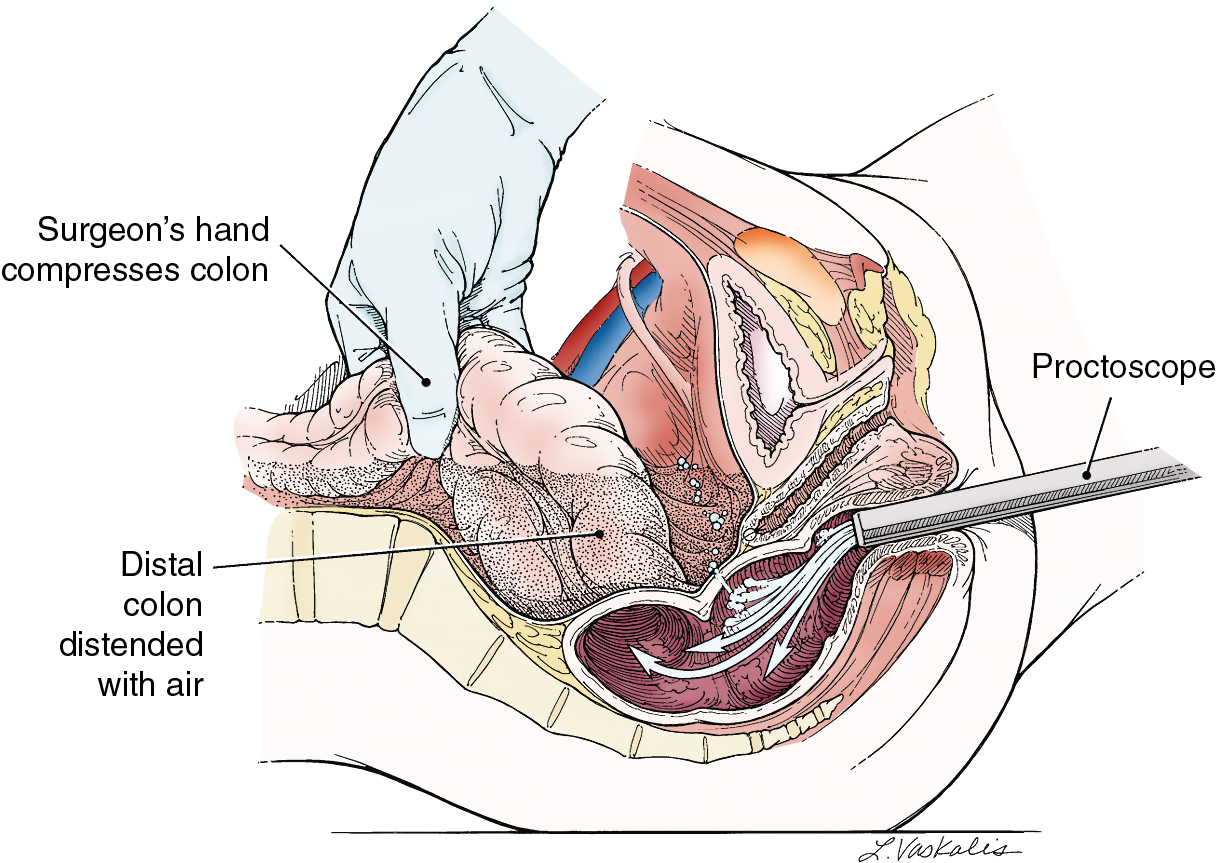
Injury to the colon most commonly occurs in the pelvis, and the risk is increased in the face of prior pelvic surgery, cancer involving the colon, or prior radiation therapy. If colonic injury occurs, the decision must be made as to whether a primary closure is sufficiently safe or whether the fecal stream should be temporarily diverted by performing a transverse loop colostomy or ileostomy. In most cases, primary closure without diversion may be performed, especially if the patient has had a mechanical bowel preparation. The principles for closure require viable tissue and no tension. In patients who have had prior pelvic radiation, the risk of perforation of the colotomy closure is significantly increased, and diversion is usually advised. After a two-layered closure of a rectosigmoid injury, the authors routinely perform proctoscopy and a “bubble test” ( Fig. 13.8 ). If either test suggests a defect in the anastomosis, a diverting ostomy is recommended.
Postoperative gastrointestinal complications
Ileus.
After abdominal or pelvic surgery, most patients experience some degree of intestinal ileus. The exact mechanism by which this arrest and disorganization of GI motility or obstipation occurs is unknown. It appears to be associated with the opening of the peritoneal cavity and is aggravated by manipulation of the intestinal tract and prolonged surgical procedures. Ileus is much less commonly associated with minimally invasive surgical procedures. If ileus were to occur after laparoscopy or robotic surgery, the surgeon should strongly consider a more serious bowel injury and evaluate the patient accordingly. Infection, peritonitis, postoperative hypervolemia causing bowel wall edema, and electrolyte disturbances may also result in ileus. Perioperative use of opioids can also contribute to ileus development. For most patients undergoing gynecologic cancer operations, the degree of ileus is minimal and GI function returns relatively rapidly, allowing the resumption of oral intake within a few days of surgery. Patients who have persistently diminished bowel sounds, abdominal distention, and nausea and vomiting require further evaluation and more aggressive management.
Ileus is usually manifest by abdominal distention and should be evaluated initially by physical examination, assessing the quality of bowel sounds, searching for tenderness or rebound on palpation, and evaluating for tympany. The possibility that the patient’s symptoms and examination findings may be associated with a more serious intestinal obstruction or other intestinal complication must be considered. Pelvic examination should be performed to evaluate for the presence of a pelvic abscess or hematoma that can cause or contribute to an ileus. Abdominal radiography to evaluate the abdomen in the supine position and in the upright position usually will aid in the diagnosis of an ileus. The most common radiographic findings of an ileus include dilated loops of small and large bowel and air–fluid levels in the upright position. In postoperative patients, especially in the upright position, the abdominal x-ray may also show evidence of free air. This is a common finding after surgery; it lasts 7 to 10 days in some instances and is not indicative of a perforated viscus in most patients. Acute colonic pseudo-obstruction (Ogilvie syndrome) should be considered in the differential diagnosis.
The initial management of a postoperative ileus is aimed at GI tract decompression and maintenance of appropriate IV replacement fluids and electrolytes.
- 1.
If a patient has moderate to severe vomiting, not improved with bowel rest and antiemetics, an NG tube should be placed to evacuate the stomach of its fluid and gaseous contents. Prolonged NG suction continues to remove swallowed air, which is the most common source of air in the small bowel.
- 2.
Fluid and electrolyte replacement must be adequate to keep the patient well perfused. Significant amounts of third-space fluid loss occur in the bowel wall, the bowel lumen, and the peritoneal cavity during the acute episode. GI fluid losses from the stomach (vomiting or NG suction) may also lead to a metabolic alkalosis and depletion of other electrolytes. Careful monitoring of serum chemistries and appropriate replacement are necessary.
- 3.
Most cases of severe ileus begin to improve over a period of several days. In general, this is recognized by reduction in the abdominal distention, return of normal bowel sounds, and passage of flatus or stool. Follow-up abdominal radiographs may be obtained as necessary for further monitoring.
- •
When the GI tract function appears to have returned to normal, the NG tube may be removed, and the patient can be started on a liquid diet to be advanced as tolerated.
- •
If a patient shows no evidence of improvement during the first 48 to 72 hours of medical management, other causes of ileus should be sought. Such cases may include ureteral injury, peritonitis from pelvic infection, unrecognized GI tract injury with peritoneal spill, or fluid and electrolyte abnormalities such as hypokalemia. In the evaluation of persistent ileus, the use of water-soluble upper GI contrast studies may assist in the resolution of the ileus, the recognition of a small bowel obstruction, or injury (leak) of the GI tract.
- •
Small bowel obstruction.
Obstruction of the small bowel after abdominal surgery occurs in approximately 1% to 2% of patients, but it may be more common after radical gynecologic oncology procedures as a result of the extensive dissection and manipulation of the small bowel. This complication is less common in patients who have undergone minimally invasive surgery. The most common cause of small bowel obstruction is adhesions to the operative site. If the small bowel becomes adherent in a twisted position, partial or complete obstruction may result from distention, ileus, or bowel wall edema. Less common causes of postoperative small bowel obstruction include entrapment of the small bowel into an incisional hernia and an unrecognized defect in the small bowel or large bowel mesentery. Early in its clinical course, a postoperative small bowel obstruction may exhibit signs and symptoms identical to those of ileus. Initial conservative management, as outlined for the treatment of ileus, is appropriate. Because of the potential for mesenteric vascular occlusion and resulting ischemia or perforation, worsening symptoms of abdominal pain, progressive distention, fever, leukocytosis, or acidosis should be evaluated carefully because immediate surgery may be required.
In most cases of postoperative small bowel obstruction, the obstruction is only partial and usually resolves with conservative management.
- •
After several days of conservative management (decompression with NG tube, fluid and electrolyte replacement), further investigation may be necessary. Evaluation of the GI tract with barium enema and an upper GI series with small bowel follow-through is appropriate. Alternatively, an abdominal and pelvic CT scan with GI contrast may be useful in identifying the location of obstruction (“transition point”) and, simultaneously, evaluates for the presence of an abscess, lymphocele, or ureteral injury. In most cases, complete obstruction is not documented, although a narrowing or tethering of the segment of small bowel may indicate the site of the problem.
- •
Further conservative management with NG decompression and IV fluid replacement may allow time for bowel wall edema or torsion of the mesentery to resolve.
- •
If resolution is prolonged and the patient’s nutritional status is marginal, the use of TPN should be considered.
Conservative medical management of postoperative small bowel obstruction usually results in complete resolution; however, if persistent evidence of small bowel obstruction remains after full evaluation and an adequate trial of medical management, surgical exploration may be necessary to evaluate and manage the obstruction. In most cases, lysis of adhesions is all that is required, although a segment of small bowel that is badly damaged or extensively sclerosed from adhesions may require resection and anastomosis.
Colonic obstruction.
Postoperative colonic obstruction after gynecologic oncology surgery is exceedingly rare. Advanced ovarian carcinoma is the most common cause of colonic obstruction in postoperative gynecologic surgery patients and is caused by extrinsic impingement on the colon by the malignancy. Occult intrinsic colonic lesions (e.g., colon cancer) also may cause obstruction. When colonic obstruction is manifest by abdominal distention and abdominal radiographs reveal a dilated colon and enlarging cecum, further evaluation of the large bowel is required by barium enema or colonoscopy. Dilation of the cecum to more than 10 cm to 12 cm in diameter, as viewed by abdominal radiography, requires immediate evaluation because there is a higher probability of colonic perforation. When there is no logical cause for a mechanical obstruction, acute colonic pseudo-obstruction (Ogilvie syndrome) should be considered. Colonoscopy should be performed, and the colonic gas should be evacuated. If a true mechanical obstruction is documented, surgical decompression by performing a colectomy, colostomy, or cecostomy may be required on an urgent basis. In some circumstances, an intraluminal stent may be placed endoscopically. Surgery should be performed as soon as the obstruction is documented. Conservative management of colonic obstruction is not appropriate because the complication of colonic perforation has an exceedingly high mortality rate .
Diarrhea.
Episodes of diarrhea often occur after abdominal and pelvic surgery as the GI tract returns to its normal function and motility; however, prolonged and multiple episodes may represent a pathologic process such as impending small bowel obstruction, colonic obstruction, or pseudomembranous colitis. Excessive amounts of diarrhea should be evaluated by abdominal radiographs and stool samples to test for the presence of ova and parasites, bacterial culture, and Clostridium difficile toxin. Proctoscopy and colonoscopy may also be advisable in severe cases. Evidence of intestinal obstruction should be managed as outlined previously. Infectious causes of diarrhea should be managed with the appropriate antibiotics and fluid and electrolyte replacement. C. difficile –associated pseudomembranous colitis may result from recent exposure to any antibiotic. Discontinuation of these antibiotics (unless they are needed for another severe infection) is advisable, along with the institution of appropriate therapy. Because treatment failure associated with oral metronidazole is more common (up to 50%), the authors typically institute therapy with oral vancomycin or fidaxomicin for mild to moderate cases and oral vancomycin for severe cases. Therapy should be continued until the diarrhea abates, and several weeks of oral therapy may be required to obtain complete resolution of the pseudomembranous colitis. In cases of recurrent infection that fails to resolve with antibiotic therapy, fecal microbiota transplantation (stool transplant) is recommended.
Perforation and fistula.
GI fistulas are relatively rare after gynecologic oncology surgery. They are most often associated with advanced malignancy, prior radiation therapy, an unrecognized surgical injury to the large or small bowel, or a leak from an anastomosis. Signs and symptoms of GI fistula are often similar to those of small bowel obstruction or ileus except that a fever is more frequently a prominent component of the patient’s symptoms. When fever is associated with postoperative GI dysfunction, evaluation should include early assessment of the GI tract for its continuity. When fistula is suspected, the use of water-soluble GI contrast material is advised to avoid the complication of barium peritonitis. Evaluation with abdominal pelvic CT scan may also assist in identification of a fistula and associated abscess or fluid collection. Recognition of an intraperitoneal GI leak or fistula formation usually requires immediate surgery unless the fistula has drained spontaneously through the abdominal wall or vaginal cuff .
An enterocutaneous fistula arising from the small bowel and draining spontaneously through the abdominal incision should be suspected if bilious output or stool is noted and may be managed successfully with medical therapy. Therapy should include NG decompression, replacement of IV fluids, nutritional support (which may require TPN), wound care, and appropriate antibiotics to treat an associated mixed bacterial infection. If the infection is controlled and there are no other signs of peritonitis, the surgeon may consider allowing potential resolution of the fistula over a period of up to 6 weeks. Some authors have suggested the use of somatostatin or cholestyramine to decrease output and allow earlier healing of the fistula. In many cases, especially if the bowel was not radiated, the fistula will close spontaneously with this management. If the enterocutaneous fistula does not close with conservative medical management, surgical correction with resection, bypass, or reanastomosis will be necessary in most cases .
A rectovaginal fistula that occurs after gynecologic surgery is usually the result of surgical trauma that may have been aggravated by the presence of extensive adhesions or tumor involvement of the rectosigmoid colon and posterior cul de sac. Low rectal anastomoses are also at risk to break down and lead to a rectovaginal fistula. Perforation of a colonic diverticulum is another possible cause for a colovaginal fistula. A small rectovaginal fistula may be managed with a conservative medical approach in the hope that decreasing the fecal stream will allow closure of the fistula. At that point, usually several months later, surgical correction of the fistula is appropriate. Larger rectovaginal fistulas that have no hope of closing spontaneously are best managed by performing an initial diverting colostomy followed by repair of the fistula after inflammation has resolved. After the fistula closure is healed, the colostomy may be taken down and closed .
Perforation.
Intestinal perforation or fistula can be an adverse event caused by the use of bevacizumab, a monoclonal antibody to vascular endothelial growth factor (VEGF). More recent studies among ovarian cancer patients show between a 1% and 3% risk of GI perforation with the use of bevacizumab, a decrease from earlier studies owing, in part, to more rigorous study design and more careful patient selection. GI perforation should be suspected in patients who develop acute abdominal pain in the setting of receiving bevacizumab. Imaging may include an abdominal radiograph (to assess for the presence of free air) or CT. Unstable patients will likely need to undergo surgical management emergently. In stable patients, nonoperative management is often preferred. This may include bowel rest, TPN, antibiotics, and the placement of a percutaneous drain. If surgery is necessary in patients initially treated with conservative management, the authors recommend a delay in surgery of at least 6 weeks.
Given the risk of bevacizumab-related adverse events, such as poor wound healing, the authors recommend delaying elective surgery for 4 to 6 weeks after the last dose of bevacizumab (and another 4 to 6 weeks to reinitiate therapy).
Venous thromboembolic complications
Patients with cancer are recognized to be at a nine-fold increased risk of developing venous thromboembolism (VTE) when compared with the general population. Venous thromboembolic complications most often are identified following gynecologic oncology surgery, but they may also precede the diagnosis of gynecologic cancer or be the result of cancer treatments, such as chemotherapy.
Risk factors
The causal factors of venous thrombosis were first proposed by Virchow in 1858 and include a hypercoagulable state, venous stasis, and vessel endothelial injury. In addition to the increased risk of VTE resulting from cancer, other clinical risk factors include: advanced age, major surgery, non-White race, a history of deep vein thrombosis (DVT) or pulmonary embolism (PE), lower extremity edema or venous stasis changes, presence of varicose veins, obesity, a history of radiation therapy, hypercoagulable states (such as factor V Leiden), pregnancy, or use of oral contraceptives, estrogens, or tamoxifen. Intraoperative factors associated with postoperative DVT included increased anesthesia time, increased blood loss, and the need for transfusion in the operating room. It is important to recognize these risk factors in order to provide the appropriate level of venous thrombosis prophylaxis. The modified Caprini risk assessment model for VTE has been widely adopted as a predictive tool for surgical patients; however, this tool has limited use in the gynecologic oncology population, since nearly all patients have risk factors that place them into the “highest risk” category. In addition to assessing VTE risk, the risk of bleeding should be assessed since most prophylactic strategies involve the use of anticoagulants. In general, the risk of bleeding for patients undergoing surgery for gynecologic malignancies is approximately one percent.
Prophylactic methods
DVT and PE, although largely preventable, are significant complications in women with gynecologic cancers and especially those who are postoperative. The magnitude of this problem is relevant to gynecologic oncologists because 40% of all deaths after gynecologic surgery are directly attributed to pulmonary emboli .
Several prophylactic methods have been shown to significantly reduce the incidence of DVT in women with gynecologic cancers, and a few studies have included a large enough patient population to demonstrate a reduction in fatal pulmonary emboli. The ideal prophylactic method would be effective, free of significant side effects, well accepted by the patient and nursing staff, widely applicable to most patients, and inexpensive. Available prophylactic methods may be divided into pharmacologic agents that reduce hypercoagulable states and mechanical methods that reduce stasis and may also enhance fibrinolysis. A key to the successful use of prophylactic methods is the understanding that women with gynecologic cancers are at very high risk of VTE and that more intense prophylactic measures are necessary to achieve maximal success. Based on the American College of Chest Physician Guidelines, all high-risk patients should receive either pharmacologic anticoagulation or combination pharmacologic anticoagulation and mechanical device prophylaxis. In addition, extended prophylaxis after surgery is recommended for patients undergoing major abdominal or pelvic surgery for cancer.
Pharmacologic venous thromboembolism prophylaxis.
Numerous prospective randomized clinical trials have demonstrated the perioperative administration of “low-dose unfractionated heparin” (LDH) and low-molecular-weight heparin (LMWH) significantly reduces the incidence of postoperative VTE. More than 25 controlled trials have demonstrated that LDH, given subcutaneously 2 hours preoperatively and every 8 hours postoperatively, is effective in reducing the incidence of postoperative DVT .
Following the early trials of LDH, LMWHs were developed and shown also to be effective in reducing postoperative VTE. When compared with LDH, LMWHs have more anti-Xa and less antithrombin activity, leading to less effect on PTT and possibly to fewer bleeding complications. An increased half-life of 4 hours results in increased bioavailability when compared with LDH. The increase in half-life of LMWHs also allows the convenience of once-a-day dosing.
Randomized controlled trials (RCTs) have compared LMWH with LDH in patients undergoing gynecologic surgery and demonstrated a similar incidence of DVT with either therapy. Bleeding complications appear to be similar between the LDH and LMWH groups, although a meta-analysis of all studies that recorded the incidence of heparin-induced thrombocytopenia (HIT) found that HIT is significantly less common with LMWH. It is recognized that the increased risk of VTE continues for at least a month following surgery. To address this problem, randomized trials have shown that continued prophylaxis with LWMH following discharge results in improved outcomes in high-risk surgical populations. Pharmacologic prophylaxis for 4 weeks postoperatively has been adopted as the standard of care by the American College of Chest Physicians (ACCP), the National Comprehensive Cancer Network, and the American Society of Clinical Oncology.
Oral agents.
Oral agents including warfarin (vitamin K antagonist or VKA), aspirin, and direct oral anticoagulants (DOACs—direct thrombin inhibitors [e.g., dabigatran] and factor Xa inhibitors [e.g., rivaroxaban, edoxaban, and apixaban]) have been studied in orthopedic patients for VTE prevention; however, their use in gynecologic oncology surgery (and general surgery) have been studied in only a few trials to date, and are not typically administered in nonorthopedic surgical patients.
Mechanical methods
Actively reducing venous stasis in the legs will also decrease the incidence of postoperative VTE.
Although probably of only modest benefit, reduction of stasis by short preoperative hospital stays and early postoperative ambulation should be encouraged for all patients.
The use of calf-length or thigh-length stockings, which have a graduated level of compression decreasing proximally from the ankle, have been shown to be of modest benefit when they are carefully fitted; however, poorly fitted stockings may be hazardous to some obese patients who develop a tourniquet effect at the knee or midthigh. The simplicity of elastic stockings and the absence of significant side effects are probably the two most important reasons that they are often included in routine postoperative care.
More effective in reducing postoperative VTE are devices that provide intermittent external compression of the leg by pneumatically inflated sleeves placed around the calf or leg. Calf compression during and after gynecologic surgery significantly reduces the incidence of DVT on a level similar to that of low-dose heparin .
The optimal duration of external pneumatic compression (EPC) use has not been studied. It is the authors’ practice to continue to use EPC throughout the patient’s hospital stay when she is in bed.
One advantage of EPC is that it has no significant side effects or risks and is considered slightly more cost effective compared with pharmacologic methods of prophylaxis. EPC is generally recommended for patients who are at high risk of bleeding from pharmacologic prophylaxis. Of course, compliance to wearing the leg compression while in bed is of utmost importance, and the patient and nursing staff should be educated to the proper regimen for maximum benefit.
Given that most gynecologic oncology patients are at highest risk to develop postoperative VTE, the combined use of a pharmacologic agent and anti-stasis devices (graded compression stockings and EPC) has been advocated throughout the hospital stay. Although there have been no randomized trials on ECP alone versus combination prophylaxis specifically within gynecologic oncology populations, high-quality data from other cancer surgical populations demonstrate improved effectiveness with combination prophylaxis and have led to consensus recommendations that we follow. Mechanical prophylaxis alone should be reserved for perioperative gynecologic oncology patients with an unacceptably high bleeding risk .
Extended postoperative prophylaxis
Patients undergoing abdominal or pelvic surgery for malignancy remain at increased risk for development of VTE for an extended period of time after surgery. As noted previously, the administration of LMWH for 4 weeks postoperatively has been consistently shown to decrease development of VTE in this high-risk population. For all patients undergoing laparotomy for gynecologic cancer surgery, we recommend extended (4 weeks) pharmacologic prophylaxis with LMWH . Currently, the use of DOACs for long-term prophylaxis are undergoing clinical trials and are not recommended by the authors.
Evidence for extended VTE prophylaxis for patients undergoing minimally invasive procedures is less clear. The authors recommend extended prophylaxis after minimally invasive surgery only for patients with other conferring risk factors (i.e., limited mobility, morbid obesity, history of VTE).
Management of deep vein thrombosis and pulmonary embolism
Diagnosis of deep vein thrombosis.
Because PE is the leading cause of death after gynecologic surgical procedures, identification of high-risk patients and the use of prophylactic VTE regimens are essential parts of perioperative management; however, VTE prophylactic methods are not fool proof and postoperative VTE will still occur. Therefore, it is critical to quickly recognize a DVT or PE and provide immediate treatment.
The signs and symptoms of DVT of the lower extremities include pain, edema, erythema, and prominent vascular pattern of the superficial veins. These signs and symptoms are relatively nonspecific; 50% to 80% of patients with these symptoms do not actually have DVT. Conversely, approximately 80% of patients with symptomatic pulmonary emboli have no signs or symptoms of thrombosis in the lower extremities. Because of the lack of specificity when signs and symptoms are recognized, additional diagnostic tests should be performed to establish the diagnosis of DVT and/or PE.
Compression ultrasonography (CUS) with doppler is currently the most common technique for the diagnosis of symptomatic venous thrombosis, especially when it arises in the proximal lower extremity. It should be recognized that CUS may not detect thrombi located in the calf, isolated iliac vein, or proximal femoral vein.
In situations where ultrasound is inconclusive, other diagnostic studies may be necessary. Magnetic resonance venography (MRV) or contrasted CT venography (CTV) should be considered. Further, MRV or CTV may detect thrombi in pelvic veins, ovarian veins, or vena cava that are not identified by ultrasound. The primary drawback to MRV or CTV is the time involved in examining the lower extremity and pelvis, risk of renal injury from IV contrast, and the expense of this technology.
Measuring D-dimer values is often part of a diagnostic algorithm in the evaluation of suspected DVT; however, D-dimer is nonspecific and is often elevated in patients with cancer or recent surgery, leading to false-positive results.
Diagnosis of pulmonary embolism.
Many of the signs and symptoms of PE are associated with other, more commonly occurring pulmonary complications after surgery. The classic findings of pleuritic chest pain, hemoptysis, shortness of breath, tachycardia, and tachypnea should alert the physician to the possibility of a PE. Many times, though, the signs are much more subtle and may present only as persistent tachycardia, a slight elevation in the respiratory rate, or a decrease in oxygen saturation. Several diagnostic algorithms have been devised to aid in estimating the likelihood of PE before definitive imaging, such as the Wells or Geneva scoring systems. Interestingly, these algorithms have been compared against physician clinical estimations in a meta-analysis of 52 studies including 55,268 patients. Clinical acumen has comparable sensitivity (0.85 vs. 0.84 to 0.91) to structured diagnostic algorithms when both are combined with D-dimer testing. Patients with suspected PE can be evaluated initially by chest radiography, electrocardiography, and arterial blood gas assessment; however, a high clinical suspicion or high likelihood based on diagnostic algorithms should prompt immediate CT angiogram of the chest for definitive diagnosis. Ventilation-perfusion lung scan is an option in the setting of renal insufficiency although a high percentage may be interpreted as “indeterminate.” In this setting, careful clinical evaluation and judgment are required to decide whether pulmonary arteriography should be obtained to document or exclude the presence of a PE.
Treatment of deep vein thrombosis.
Acute anticoagulation : The treatment of postoperative DVT requires the immediate institution of anticoagulant therapy. Untreated, symptomatic DVT is associated with a 50% risk of PE, and untreated PE carries a 30% risk of mortality. Treatment may be with either unfractionated heparin (UFH), LMWHs, DOACs followed by 3 months of an oral anticoagulant (VKA, DOACs), or subcutaneous LMWH. Depending on the patient’s overall status, treatment of DVT may be initiated in the outpatient setting.
Prolonged anticoagulation (lifetime) is recommended for women who continue to have active cancer (i.e., those not in cancer remission after treatment) because these patients remain at very high risk for recurrent VTE. In this setting, evidence supports the use of either LMWH or DOCAs, which appear to be more effective and safer when compared to warfarin.
Low-molecular-weight heparin.
LMWHs (e.g., enoxaparin, dalteparin) have been shown to be effective in the treatment of VTE and have a cost-effectiveness advantage over IV heparin in that they may be administered in the outpatient setting and do not require laboratory monitoring. Monitoring of anti-Xa activity (except in difficult cases or those with renal impairment) has not been shown to be of significant benefit in the dose adjustment of LMWHs. The increased bioavailability and longer half-life associated with LMWHs allows for once-a-day or twice-a-day dosing, making outpatient management a feasible option.
Unfractionated heparin.
UFH may be the initial treatment of choice in a postoperative patient or other clinical settings in which a significant bleeding risk is appreciated and the need for immediate reversal could be necessary. An initial bolus is given intravenously followed by a continuous infusion, adjusted to therapeutic aPTT levels. Weight-based nomograms to aid in appropriate dosing are widely available and often embedded in hospital pharmacy administration protocols.
Doacs and warfarin.
Due to the slow onset of therapeutic anticoagulation, warfarin and other DOACs alone are not appropriate for the immediate treatment of DVT.
Long-term anticoagulation.
For most cases of uncomplicated DVT, anticoagulation is recommended for 3 months; however, patients with active malignancy remain at high risk to develop subsequent VTE once anticoagulation is discontinued. In this group of patients, anticoagulation with no specific endpoint is generally recommended.
Following initial parenteral treatment (LMWH or UFH), continued treatment may be transitioned to LMWH or oral agents. Traditionally, oral anticoagulation with warfarin was used in the long-term treatment of DVT in cancer patients; however, warfarin may be difficult to safely administer to some patients, especially if their nutrition is inadequate, their oral intake is variable, or they require prolonged use of antibiotics or other drugs that might alter the metabolism of warfarin. This is particularly common in women with advanced ovarian cancer. A number of large, high-quality RCTs have demonstrated the superiority of LMWHs over warfarin in long-term anticoagulation in a patient with cancer. In each study, LMWHs resulted in equal or reduced risk of recurrent DVT with no increase in bleeding events, either major or minor. More recently, DOACs have been compared to LMWH in the long-term treatment of VTE in cancer patients. In summary, DOACs have a slightly lower risk of recurrent VTE and a slightly higher risk of bleeding. Neither is statistically significant and therefore the authors recommend either LMWH or DOACs as the first-line therapy for long-term treatment of VTE in gynecologic cancer patients .
Inferior vena cava filter.
Placement of an inferior vena cava (IVC) filter is rarely recommended in the management of VTE. In the gynecologic oncology population, acute DVT may often be diagnosed in the immediate pre- or postoperative setting, when pharmacologic anticoagulation may be contraindicated because of bleeding risks. In these cases, placement of a retrievable (i.e., temporary) IVC filter is an alternative strategy to reducing the risks of PE associated with an untreated DVT. The filter can remain in place until bleeding risks decrease, at which time the patient should be started on an appropriate anticoagulation regimen. Filter retrieval is associated with a high technical success rate (85%) and should be pursued given that long-term filter placement is associated with IVC thrombosis, increased recurrent DVT, and the potential for filter migration.
Pulmonary embolism.
The treatment of PE is similar to that of acute DVT in the hemodynamically stable patient. UFH, LMWH, or fondaparinux are all initial parenteral anticoagulation treatment options. Recommendations from the ACCP currently favor LMWH or fondaparinux for initial treatment, although UFH may be more prudent for the gynecologic oncology patient for whom a perioperative status or high disease burden often confers a bleeding risk requiring easy reversal of anticoagulation method. Hemodynamically unstable patients require immediate care in an ICU and assessment for thrombolytic therapy (e.g., recombinant tissue type plasminogen activator, streptokinase) or embolectomy if thrombolysis is contraindicated.
Superior vena cava syndrome.
Superior vena cava syndrome is caused by advanced cancers arising in or invading the mediastinum, subsequently obstructing the venous drainage of the head, neck, and upper thoracic regions. Primary tumors are most commonly the cause of this syndrome (bronchogenic carcinomas), although metastasis to the mediastinum from gynecologic cancers can also present in this manner. The vena cava has a low intravascular pressure and is easily compressed by adjacent masses. Most commonly, the symptoms caused by venous obstruction are dramatic swelling and plethora of the head, neck, upper extremities, and chest. Pleural and pericardial effusions can occur with decreased venous return to the heart and a resultant decrease in cardiac output. Patients also commonly complain of a severe headache. A similar clinical syndrome is also seen associated with thrombosis of the subclavian vein and superior vena cava, associated with central venous catheters. The diagnosis of the cause of superior vena cava syndrome is critical to selecting proper management. If a localized primary or metastatic neoplasm is identified, immediate radiation therapy is usually the most effective method to achieve resolution. Radiation therapy to the mediastinum in doses of 400 cGy for 3 days and then 150 to 180 cGy per day for a total dose of 3000 to 5000 cGy has been successful in relieving the vascular obstruction. Responses are commonly recognized in 3 to 4 days. Chemotherapy may also play a role, although the resolution of symptoms is usually much slower. Expandable wire stents across the constricted portion of the vena cava have also been used successfully.
In patients in whom thrombosis is the etiology of venous obstruction, immediate anticoagulant therapy should be instituted ( Fig. 13.9 ). The edema and plethora will usually diminish in 1 to 3 days. In many instances, the central venous catheter may be left in place and used. However, if the condition persists or recurs, the catheter should be removed.