Key Points
- 1.
Women with gynecologic cancers can have significant complications from treatment.
- 2.
Vaginal bleeding is usually controlled with packing.
- 3.
Ureteral obstruction can be treated by diversion either with surgery or stent placement.
- 4.
Bowel complications are treated with conservative management or surgery depending on the patient’s disease status and overall condition.
- 5.
Deep vein thrombosis always needs to be considered for prophylaxis and treatment if necessary.
- 6.
Infectious complications always need to be considered and managed appropriately.
- 7.
The risk of complications always needs to be considered and the patient counseled appropriately.
Women with gynecologic cancers often experience complications associated with their primary disease process or from the cancer-directed treatment modalities. In addition, many women have medical comorbidities, are obese, or are elderly, all of which further complicate therapy and treatment decisions. Minimizing these problems requires the clinician to astutely evaluate the patient, be proactive in prevention strategies, and provide early intervention.
Complications of disease are, in fact, commonly the primary presenting symptom (chief complaint) of a gynecologic cancer. Common symptoms of disease include hemorrhage (cervical and endometrial cancers), urinary tract obstruction or fistulae (cervical cancer), and intestinal obstruction or weight loss (ovarian cancer). Although some complications have been discussed previously in this text, it seems appropriate to devote a chapter exclusively to complications of disease and therapy. Not all possible complications can be covered, and readers are referred to texts that expand on them. However, the most common complications and management are discussed.
Disease–Oriented Complications
Symptoms caused by cancer, such as bleeding, urinary tract obstruction, fistula, and intestinal obstruction, are complications of the primary gynecologic cancer that usually need to be managed coincidentally with the cancer itself.
Hemorrhage
Bleeding from cervical or endometrial cancer is a common presenting symptom. Although bleeding is rarely severe, the acute management of hemorrhage may be required before cancer therapy can be undertaken. Patients who are bleeding should be initially assessed for hemodynamic stability. On rare occasions, the bleeding is so severe that the patient may be in hypovolemic shock. Immediate management should include venous access, blood volume replacement, and supportive care. When stabilized, the patient should be examined, and the source of the bleeding should be determined. Most commonly, massive hemorrhage results from an exophytic cervical cancer eroding into a small cervical or vaginal artery. Prolonged slow vaginal bleeding from an endometrial cancer or sarcoma may also result in a patient presenting with profound chronic anemia. Because the bleeding has been slow over a longer period, the patient has often adapted to the anemia and may be hemodynamically stable despite profound anemia. Biopsy should be performed to document the pathology, and the patient should be evaluated to make a clinical estimation of the extent (stage) of disease.
Control of an actively bleeding cervical lesion is usually accomplished with a vaginal pack applied firmly to the cervix, filling the entire vagina. Monsel’s solution (ferric subsulfate) may be put on the portion of the pack abutting the tumor. Soaking of the entire pack with Monsel’s solution should be avoided because it will desiccate the normal vaginal mucosa, making removal of the pack and subsequent pelvic examinations difficult. Application of acetone to the pack adjacent to the tumor has also been helpful, although acetone is often difficult to acquire in today’s medical environment. An indwelling Foley catheter should be placed in the bladder because pressure from the pack will usually obstruct the urethra. The pack should be removed slowly 24 to 48 hours later, and the patient should be observed. Removal of the pack under anesthesia may provide a level of safety if immediate cautery or repacking was necessary. This would also provide the opportunity to perform an examination under anesthesia and cystoscopy or proctoscopy if indicated. Suturing bleeding points in a cervical cancer is rarely successful because the suture will tear through the tumor.
Pelvic radiation therapy for a patient with locally advanced cervical cancer who is actively bleeding should be initiated immediately. Alternatively, if the patient’s cancer is an operable lesion, surgery should be performed expeditiously. In either event, if the patient has received more than 4 units of packed red blood cells (PRBCs), it is prudent to assess coagulation factors because the patient may have developed a “dilutional” coagulopathy and require fresh-frozen plasma (FFP), cryoprecipitate, or platelets.
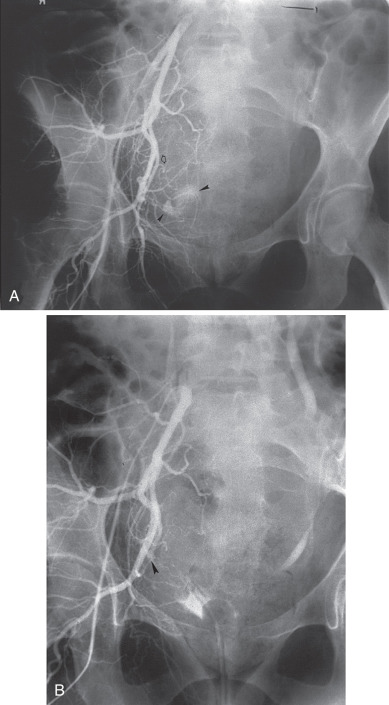
If bleeding cannot be controlled with packing, other measures must be considered. Consultation with an interventional radiologist should be obtained to consider arteriographic embolization of the hypogastric or uterine arteries. Arteriographic evaluation will usually identify the specific bleeding vessel, and selective embolization can be accomplished. Arterial access is usually obtained through the femoral artery, and the catheter is advanced to the aortic bifurcation. Using contrast injected into the artery, the arterial vascular supply of the pelvis can be investigated to identify the specific bleeding site. Both sides of the pelvis should be evaluated. Intravascular contrast can be nephrotoxic and therefore must be used cautiously in patients who have an element of renal failure or who have diabetes. Control of the bleeding site can be accomplished by continuous vasopressin infusion, by embolization using synthetic materials (Gelfoam) or Gianturco springs imbedded with Dacron or with a balloon catheter ( Fig. 16.1 ). Embolization is usually the procedure primarily chosen because the vasopressin infusion and balloon catheters require that the artery remain cannulated for a longer duration.
Hypogastric (internal iliac) artery ligation is usually the procedure of last resort for bleeding from a primary gynecologic cancer and is most commonly performed to control intraoperative hemorrhage. Details of hypogastric artery ligation are discussed later in this chapter.
Urinary Tract Complications
Ureteral Obstruction
Ureteral obstruction may be the primary presenting symptom of a locally advanced cervical cancer and less commonly other gynecologic cancers, including endometrial and ovarian cancers. The most common evidence of ureteral obstruction is an elevated serum creatinine level (rather than complaints of anuria or symptoms of uremia). Of course, acute renal failure may arise from a number of causes, which should be investigated ( Table 16.1 ). The ureters may be obstructed as a result of local extension of the cancer, by metastases to retroperitoneal lymph nodes, or by extrinsic compression of the ureter by large masses. Uremia secondary to bilateral ureteral obstruction is rarely encountered today but warrants immediate recognition and treatment. Given evidence of an elevated creatinine, evaluation of the ureters should avoid the use of nephrotoxic intravenous (IV) contrast dyes. A noncontrasted computed tomography (CT) scan of the abdomen and pelvis can demonstrate suspected hydronephrosis and provides the additional benefit of evaluating disease burden. Alternative imaging methods may include ultrasonography of the kidneys or a Lasix-renal scan to access if the kidney remains viable. If bilateral ureteral obstruction is diagnosed, the patient should be rapidly evaluated to determine the true extent of the cancer before any intervention is undertaken. If the cancer appears to be locally advanced and not widely metastatic, relief of the ureteral obstruction should be attempted by cystoscopy and placement of retrograde ureteral stents. If stent placement is unsuccessful, then percutaneous nephrostomy (PCN) tubes should be inserted. Dialysis may be necessary in extreme circumstances until the obstruction can be relieved. Postobstructive diuresis and correction of electrolytes should be carefully evaluated in the several days after relief of the ureteral obstruction.
| Disorder | Example |
|---|---|
| PRERENAL FAILURE | |
| Hypovolemia | Skin, gastrointestinal, or renal volume loss; hemorrhage, sequestration of extracellular fluid (pancreatitis, peritonitis) |
| Cardiovascular failure | Impaired cardiac output (infarction, tamponade); vascular pooling (anaphylaxis, sepsis, drugs) |
| POSTRENAL FAILURE | |
| Extrarenal obstruction | Urethral occlusion; bladder, pelvic, or retroperitoneal neoplasms; surgical accident; calculi |
| Intrarenal obstruction | Crystals (uric acid, oxalic acid, sulfonamides, methotrexate) |
| Bladder rupture | Trauma |
| ACUTE TUBULAR NECROSIS | |
| Postischemic | All conditions listed above for prerenal failure |
| Pigment induced | Hemolysis (transfusion reaction); rhabdomyolysis (trauma, coma, heatstroke, severe exercise, potassium or phosphate depletion) |
| Toxin induced | Antibiotics; contrast material; anesthetic agents; heavy metals; organic solvents |
| Pregnancy related | Septic abortion; uterine hemorrhage; eclampsia |
Complications of PCN placement include a high frequency of urinary tract infections (UTIs) and pyelonephritis (70%), catheter occlusion (65%), and bleeding (28%). Seventy percent of the patients will have recovery of renal function after PCN placement (Dudley).
Comment needs to be made on two sets of circumstances in which the physician, patient, and family must seriously consider the possibility that relief of the obstruction may not be in the patient’s best interest. These clinical situations include the following:
- •
A patient who presents with a widely metastatic malignancy for which there is little significant opportunity to provide effective therapy
- •
A patient who has previously been treated for cervical cancer and has bilateral obstruction secondary to recurrent pelvic disease. This is a situation in which there is no therapy available that would significantly prolong the patient’s life. Careful evaluation should be made to be certain that the obstruction is not a result of retroperitoneal fibrosis caused by prior radiation therapy or from a lymphocyst.
Often, patients with bilateral ureteral obstruction are uremic and comatose. Decisions regarding intervention and care then fall to the next of kin, who must make the difficult decisions regarding intervention that may reverse the uremia but cause the patient to succumb from other complications of the cancer versus allowing the patient to expire peacefully in a uremic coma. Compassionate and knowledgeable consultation and advice with an experienced gynecologic oncologist are crucial in these difficult circumstances.
Unilateral ureteral obstruction at the time of initial presentation may not require stent or PCN placement if the patient’s renal function is normal and therapy (eg, pelvic radiation therapy) is expected to control the cancer and relieve the obstruction. Placement of a PCN or stent in these circumstances must be balanced against the potential complications that might delay or interrupt therapy ( Fig. 16.2 ). A Lasix renal scan can aid in evaluating the function of the obstructed kidney. If the organ is still viable, placement of a stent to avoid loss of function while awaiting tumor shrinkage from therapy can be helpful.
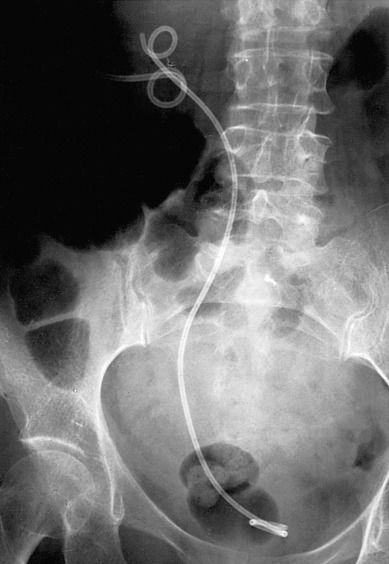
Urinary outlet obstruction (obstruction of the urethra) by a cancer that has invaded the anterior vaginal wall (vaginal, vulvar, or cervical cancers) may usually be corrected by placement of a Foley catheter. If a Foley catheter cannot be placed, either a suprapubic catheter or PCNs should be considered.
Urinary Tract Fistulas
Vesicovaginal fistula caused by a primary gynecologic cancer is relatively rare and is more commonly caused by therapy. Nonetheless, some patients will present with tumor that has eroded into the bladder, and subsequent loss of integrity between the bladder and vagina results in urinary leakage. Correction of the fistula caused by a cancer cannot be considered until the cancer has been eradicated. In the interim, while cancer therapy is initiated, the patient may be very uncomfortable from the continued loss of urine. Attempts to diminish the leakage should be undertaken. Placement of a Foley catheter will often partially divert urine from the fistula into the catheter. Modified menstrual cups or external appliances to collect urine have been used on occasion with success. Urinary diversion (ileal or transverse colon conduit) may be the only complete solution to profuse vaginal urinary leakage. Performing this major surgery should be weighed against the delay in primary cancer therapy, which would be required while the patient recovers from surgical diversion.
Gastrointestinal Complications
Gastrointestinal Obstruction
Intestinal obstruction as a presenting symptom of a gynecologic cancer is most commonly caused by advanced ovarian cancer. In cases of small intestinal obstruction, initial therapy should include correction of fluid volume and electrolytes, nutritional assessment, and bowel rest. If nausea and emesis continue despite antiemetics, a nasogastric (NG) tube decompression should be placed. Alternatively, an NG tube may be placed immediately if there is significant stomach distention requiring decompression. Assessment of intestinal patency a CT scan with oral contrast should be performed to have a better understanding of the location and extent of obstruction. The colon should also be evaluated to exclude the possibility of colonic obstruction, which would need to be relieved at the same surgical procedure. In most cases, surgical exploration is necessary to establish and stage the cancer diagnosis, debulk the tumor, and relieve the obstruction. Small bowel or colonic resection performed to relieve obstruction and to debulk the primary tumor is commonly done. Although patients presenting with gastrointestinal (GI) obstruction may be severely malnourished, the role of preoperative total parental nutrition is debatable. The highest quality evidence suggests that at least 10 days of total parenteral nutrition (TPN) administration is necessary to provide a benefit of reduced postoperative complications.
Short bowel syndrome may result from extensive resection of the small bowel, colon, or both. The syndrome is characterized by frequent diarrhea, fluid and electrolyte depletion, malabsorption, and weight loss. Depending on the extent and location of the intestinal segment(s) resected, malabsorption of nutrients may include copper, zinc, chromium, selenium, essential fatty acids, vitamins A and E, biotin, thiamine, and vitamin B 12 . Over time, the remaining small bowel often adapts, and fluid and nutrient absorption is improved. However, in the interim, attempts to relieve short bowel syndrome should be directed at decreasing transit time by the use of an “elemental” diet and Imodium or Lomotil, cholestyramine (to decrease irritation of bile salts on the colonic mucosa), and somatostatin (to decrease intestinal digestive fluid production). In extreme cases, support with IV fluids and TPN may be necessary for several months.
On occasion, the preoperative assessment (usually with a CT scan) discovers far advanced disease (extensive carcinomatosis), which would be unlikely to be successfully debulked. In these patients, neoadjuvant chemotherapy, rather than surgical intervention, may be the best option. If this therapeutic strategy is taken, GI decompression (NG tube or gastrostomy) and parenteral nutritional support (TPN) will be required for several weeks while the neoadjuvant chemotherapy has the opportunity to result in a tumor response and relief of the intestinal obstruction. Fortunately, many patients with ovarian cancer will regain intestinal function after two or three cycles of chemotherapy.
Even in the setting of neoadjuvant treatment route, correction of a colonic obstruction is necessary to prevent colonic perforation, peritonitis, sepsis, and death. Management options include placement of an intraluminal stent or surgical intervention. In a recently published experience with gynecologic oncology patients, rates of technical success of stent placement ranged from 75% to 88%; however, clinical success was lower, with more than one-third (33%–38%) of patients with stent placements requiring further intervention for either stent complication or failure. Advantages of stents include the avoidance of major surgery and a stoma. Systematic review of large patient populations beyond gynecologic oncology demonstrate median placement success rates of 96%, with associated risks of perforation (4.5%), stent migration (11%), and reobstruction (12%). In a palliative population, the median duration of patency was 106 days. Alternatively, surgical correction of the colonic obstruction may be considered, with colostomy as a frequent outcome given the disease burden in a neoadjuvant patient. If the patient has an excellent response to subsequent chemotherapy, colostomy take-down in the future is reasonable to consider.
Intestinal obstruction often occurs late in the course of progressive ovarian cancer. In these situations, superb clinical judgment is required to obtain an optimal palliative outcome because not all patients with recurrent ovarian cancer and intestinal obstruction benefit from surgical intervention. It does seem intuitive that patients with colonic obstruction should be either offered a self-expanding stent or undergo surgery to create colostomy, ileostomy, or cecostomy. A patient with a small bowel obstruction requires careful thought and triage. Initially, conservative management with IV fluid and electrolyte replacement and NG tube decompression should be instituted. Some patients may reestablish bowel function with a few days of “bowel rest.” However, if the obstruction persists, the decision to place a gastrostomy tube (which can often be placed percutaneously) or to attempt to surgically relieve the intestinal obstruction must be made. (Of course, a patient who has a small bowel obstruction caused by adhesions should undergo surgery in all cases.) The problem in decision making comes when it is clear that the patient has recurrent ovarian cancer. Many investigators have attempted to identify factors that would predict successful outcome (often defined as surviving 30 days or being discharged from the hospital and able to take oral fluids) or postoperative complications and death. These factors include presence of ascites, poor nutritional status, amount of prior chemotherapy regimens, availability of therapy with some potential for response, prior use of radiation therapy, length of time since prior therapy, and potential for being “platin sensitive.” If surgical intervention is deemed appropriate, surgical procedures might include bypass of involved segments of small bowel (entero-enterostomy), bowel resection with anastomosis, or ileostomy. Unfortunately, in every investigator’s experience, there are patients who undergo laparotomy only to find such extensive carcinomatosis that they are deemed inoperable. The decision to operate, then, should be based on a clear communication between the surgeon and patient regarding expectations and definitions of “success.” In the authors’ experience, which is reasonably representative of the general literature, the median survival time after small bowel obstruction surgery was 88 days, and only 14% of patients were alive at 12 months. In addition, 49% of patients had at least one significant postoperative complication, including wound infections, enterocutaneous fistula, sepsis, and recurrent obstruction.
If the decision is made not to operate, further decisions regarding management are also complex, including methods to palliate vomiting (percutaneous gastrostomy is recommended) and whether to continue IV fluids or even consider TPN in a hospice setting.
Gastrointestinal Fistulas
Rectovaginal fistula may be discovered at the time of primary diagnosis of cervical, vaginal, or vulvar cancers. Involuntary loss of feces, flatus, and mucous discharge are the most common symptoms. If the patient has vulvar pain and excoriation, a fistula from the small intestine must be suspected. In this instance, a fistulogram can be helpful if definitive diagnosis of a fistulae is in question, and a CT scan with oral contrast should be performed to define the exact anatomic structures involved. If a rectovaginal fistula is found, diversion with a loop colostomy or end-colostomy is suggested to divert the fecal stream and allow prompt treatment of the cancer (usually radiation therapy). If vulvar cancer is so advanced as to cause a rectovaginal fistula, some surgeons manage the cancer and the fistula in the same surgical procedure (eg, a posterior pelvic exenteration and modified radical vulvectomy). Others have had excellent results treating locally advanced vulvar cancer with radiation therapy and concurrent radiosensitizing chemotherapy, thereby preserving the rectal sphincter. Colostomy diversion is still suggested for patient comfort and hygiene. If the cancer treatment is successful, attempts to close the fistula are reasonable, and if successful, the colostomy may ultimately be reversed.
Enterovaginal fistulas are rare to complicate the initial cancer diagnosis and more often occur as a result of complications of therapy (radiation) or at the time of cancer recurrence. The flow of intestinal contents out of the vagina is usually liquid and caustic to vulvar skin. Thorough evaluation of the upper and lower GI tracts and the urinary tract is mandatory because many of these fistula are “complex,” involving more than one viscus and more than one defect. Surgical intervention is necessary in most cases to either resect or isolate the involved bowel. If resection is not possible, the fistualized bowel will need to be isolated and excluded from the intestinal stream. Because the isolated bowel will continue to create succus entericus and subsequent continued vaginal drainage, resection is generally preferred.
Venous Thromboembolic Complications
Venous thromboembolic complications may precede the diagnosis of gynecologic cancer or may be the result of cancer treatments, especially surgery and chemotherapy. Most women with gynecologic cancers have several risk factors that increase the probability of developing a venous thromboembolic event during their course of therapy.
Risk Factors
The causal factors of venous thrombosis were first proposed by Virchow in 1858 and include a hypercoagulable state, venous stasis, and vessel endothelial injury. In addition to the increased risk of venous thromboembolism (VTE) resulting from cancer, other clinical risk factors include advanced age; major surgery; nonwhite race; a history of deep vein thrombosis (DVT) or pulmonary embolism; lower extremity edema or venous stasis changes; presence of varicose veins; being overweight; a history of radiation therapy; and hypercoagulable states, such as factor V Leiden, pregnancy, or use of oral contraceptives, estrogens, or tamoxifen. Intraoperative factors associated with postoperative DVT included increased anesthesia time, increased blood loss, and the need for transfusion in the operating room. It is important to recognize these risk factors in order to provide the appropriate level of venous thrombosis prophylaxis. The modified Caprini risk assessment model for VTE has been widely adopted as a predictive tool for surgical patients. This tool has limited use in the gynecologic oncology population, however. Importantly, nearly all gynecologic oncology patients, by virtue of having a malignancy, being older than 40 years old, with an operative time of more than 45 minutes are in the highest risk group, with a VTE risk of 6% in the absence of prophylaxis.
Prophylactic Methods
Deep vein thrombosis and pulmonary embolism, although largely preventable, are significant complications in women with gynecologic cancers and especially those who are postoperative. The magnitude of this problem is relevant to gynecologic oncologists because 40% of all deaths after gynecologic surgery are directly attributed to pulmonary emboli, which is also the most common cause of postoperative death in patients with uterine or cervical carcinoma.
A number of prophylactic methods have been shown to significantly reduce the incidence of DVT in women with gynecologic cancers, and a few studies have included a large enough patient population to demonstrate a reduction in fatal pulmonary emboli. The ideal prophylactic method would be effective, free of significant side effects, well accepted by the patient and nursing staff, widely applicable to most patients, and inexpensive. Available prophylactic methods may be divided into pharmacologic agents that reduce hypercoagulable states and mechanical methods that reduce stasis and may also enhance fibrinolysis. A key to the successful use of prophylactic methods is the understanding that women with gynecologic cancers are at very high risk and that more intense prophylactic measures are necessary to achieve maximal success. Based on the American College of Chest Physician Guidelines, all high-risk patients should receive either pharmacologic anticoagulation or combination pharmacologic anticoagulation and mechanical device prophylaxis. In addition, extended prophylaxis after surgery is recommended for patients undergoing major abdominal or pelvic surgery for cancer.
Low-dose heparin.
The use of small doses of subcutaneously administered heparin for the prevention of DVT and pulmonary embolism is the most widely studied of all prophylactic methods. More than 25 controlled trials have demonstrated that heparin given subcutaneously 2 hours preoperatively and every 8 to 12 hours postoperatively is effective in reducing the incidence of DVT. The value of low-dose heparin in preventing fatal pulmonary emboli was established by a randomized, controlled, multicenter international trial, which demonstrated a significant reduction in fatal postoperative pulmonary emboli in general surgery patients receiving low-dose heparin every 8 hours postoperatively. In gynecologic oncology patients, a randomized trial of heparin given either in a regimen of 5000 units subcutaneously 2 hours preoperatively and every 8 hours postoperatively or 5000 units subcutaneously every 8 hours preoperatively (a minimum of three preoperative doses) and every 8 hours postoperatively was performed. Both of these prophylaxis regimens were effective in significantly reducing the incidence of postoperative DVT in patients with gynecologic cancers. The authors conclude that in women undergoing surgery for gynecologic malignancy, a regimen of low-dose heparin 5000 units every 8 hours is necessary to provide effective prophylaxis.
The benefits of unfractionated heparin (UFH) are its rapid onset of action and ability for quick reversal; ability for frequent monitoring with activated partial thromboplastin time (aPTT) levels; and lack of renal metabolism, making it ideal in cases of renal insufficiency or renal failure. The most serious adverse risk of UFH use is the occurrence of heparin-induced thrombocytopenia (HIT). HIT has been found to be associated with low-dose heparin use in 6% of patients after gynecologic surgery, although its overall postoperative risk is considered to be around 1%. If patients remain on low-dose heparin for more than 4 days, it is reasonable to check a daily platelet count for 14 days or until heparin is stopped, whichever comes first. Low-dose heparin is also limited by a narrow therapeutic range, requiring frequent monitoring.
Low-molecular-weight heparins.
Low-molecular-weight heparins (LMWHs) are the preferred choice in perioperative pharmacologic prophylaxis. LMWHs are fragments of heparin that vary in size from 4500 to 6500 daltons. When compared with UFH, LMWHs have more anti-Xa and less antithrombin activity, leading to less effect on partial thromboplastin time (PTT) and possibly to fewer bleeding complications. An increased half-life of 4 hours results in increased bioavailability when compared with UFH. The increase in half-life of LMWHs also allows the convenience of once or twice-a-day dosing at 30 to 40 mg/day and can be used in the outpatient setting.
Randomized controlled trials (RCTs) have compared LMWH with UFH in patients undergoing gynecologic surgery. In all studies, there was a similar incidence of DVT. Bleeding complications were similar between the UFH and LMWH groups, and meta-analysis of all studies comparing the incidence of HIT confirmed that HIT is significantly less common with LMWH. A meta-analysis of general surgery and gynecologic surgery patients from 32 trials likewise indicated that daily LMWH administration is as effective as UFH in DVT prophylaxis without any difference in hemorrhagic complications, and these results have been confirmed in cancer surgery populations. Finally, prolonged prophylaxis for 4 weeks postoperatively has resulted in improved outcomes in high-risk surgical populations. In one randomized study of 1113 patients, 4 weeks of treatment after abdominal or pelvic cancer surgery reduced VTE rate from 4.6% to 0.8%, without increased bleeding complications. This extended prophylaxis has been adopted as the standard of care by the American College of Chest Physicians (ACCP), the National Comprehensive Cancer Network, and the American Society of Clinical Oncology.
Oral agents.
Investigations into the use of oral agents, specifically vitamin K antagonist warfarin, and a host of newly developed factor Xa inhibitors is ongoing. High-quality randomized trials and investigations in cancer populations have been limited to date, so the agents are not recommended in perioperative VTE prophylaxis at this time.
Mechanical Methods
Stasis in the veins of the legs has been clearly demonstrated while the patient is undergoing surgery and continues postoperatively for varying lengths of time. Stasis occurring in the capacitance veins of the calf during surgery plus the hypercoagulable state induced by cancer and surgery are the primary factors contributing to the development of acute postoperative DVT. Prospective studies of the natural history of postoperative venous thrombosis have shown that the calf veins are the predominant site of thrombi and that most thrombi develop within 24 hours of surgery.
Although probably of only modest benefit, reduction of stasis by short preoperative hospital stays and early postoperative ambulation should be encouraged for all patients. Elevation of the foot of the bed, raising the calf above heart level, allows gravity to drain the calf veins and should further reduce stasis.
Graduated compression stockings.
Controlled studies of graduated pressure stockings are limited but do suggest modest benefit when they are carefully fitted. Poorly fitted stockings may be hazardous to some obese patients who develop a tourniquet effect at the knee or midthigh. Variations in human anatomy do not allow perfect fit of all patients to available stocking sizes. There is no therapeutic advantage of thigh-length stockings as compared with calf-length stocking. The simplicity of elastic stockings and the absence of significant side effects are probably the two most important reasons that they are often included in routine postoperative care.
External pneumatic compression.
The largest body of literature dealing with the reduction of postoperative venous stasis deals with intermittent external compression of the leg by pneumatically inflated sleeves placed around the calf or leg during intraoperative and postoperative periods. Calf compression during and after gynecologic surgery significantly reduces the incidence of DVT on a level similar to that of low-dose heparin. In addition to increasing venous flow and pulsatile emptying of the calf veins, external pneumatic compression (EPC) also appears to augment endogenous fibrinolysis, which may result in lysis of very early thrombi before they become clinically significant.
The duration of postoperative EPC has differed in various trials.
External pneumatic compression used in patients undergoing major surgery for gynecologic malignancy has been found to reduce the incidence of postoperative venous thromboembolic complications by nearly threefold but only if calf compression is applied intraoperatively and for the first 5 postoperative days. Patients with gynecologic malignancies may remain at risk because of stasis and hypercoagulable states for a longer period than general surgical patients and therefore appear to benefit from longer use of EPC.
External pneumatic leg compression has no significant side effects or risks and is considered slightly more cost effective compared with pharmacologic methods of prophylaxis. Of course, compliance to wearing the leg compression while in bed is of utmost importance, and the patient and nursing staff should be educated to the proper regimen for maximum benefit. We have investigated the risk factors associated with the failure of external compression to prevent DVT in a retrospective analysis of 1862 consecutive gynecologic surgery patients who received postoperative intermittent pneumatic compression. A history of prior VTE, diagnosis of cancer, and age older than 60 years were factors independently associated with the development of VTE despite EPC prophylaxis ( P <0.05). Patients having two or more of these factors had a 16-fold increased risk of postoperative VTE despite prophylaxis. In these extremely high-risk patients, of which the majority of gynecologic oncology patients would be categorized, combined methods of prophylaxis (eg, EPC plus low-dose UFH or LMWH) is recommended by the guidelines written by the ACCP. Although there have be no randomized trials on ECP alone versus combination prophylaxis specifically within gynecologic oncology populations, high-quality data from other cancer surgical populations have led to consensus recommendations that we follow. Mechanical prophylaxis alone should be reserved for perioperative gynecologic oncology patients with unacceptably high bleeding risk.
Postoperative Prophylaxis
Patients undergoing abdominal or pelvic surgery for malignancy remain at increased risk for development of VTE for an extended period of time after surgery. The administration of LMWH for 4 weeks postoperatively has been consistently shown to decrease development of VTE in this high-risk population. For all patients undergoing laparotomy for gynecologic cancer surgery, we recommend extended (4 weeks) chemoprophylaxis with LMWH . Evidence for extended treatment for patients undergoing minimally invasive procedures is less clear. Minimally invasive approaches to hysterectomy are associated with a threefold decreased incidence of VTE. In several retrospective reviews of large ( n ≥400) institutional studies, rate of VTE after laparoscopic or robotic hysterectomy has been reported at 0.5% to 1.2%, on par with the risk associated with open benign hysterectomy (0.6%). This suggests extended prophylaxis may not be required for this patient population. Importantly, in one study of 573 cases in which the VTE rate was highest (1.2%), the authors identified a high-risk group of women with a body mass index (BMI) greater than 40 and operative time longer than 180 minutes, who had a VTE rate of 9.5% (4 of 42) compared with 0.6% (3 of 531) of the remaining population. At this time, the authors recommend extended prophylaxis after minimally invasive surgery only for patients with other conferring risk factors (ie, limited mobility, super morbid obesity, history of VTE).
Management of Deep Vein Thrombosis and Pulmonary Embolism
Diagnosis of deep vein thrombosis.
Because pulmonary embolism is the leading cause of death after gynecologic surgical procedures, identification of high-risk patients and the use of prophylactic VTE regimens is an essential part of management. In addition, the early recognition of DVT and pulmonary embolism and immediate treatment are critical. Most pulmonary emboli arise from the deep venous system of the leg, although after gynecologic surgery the pelvic veins are a known source of fatal pulmonary emboli also.
The signs and symptoms of DVT of the lower extremities include pain, edema, erythema, and prominent vascular pattern of the superficial veins. These signs and symptoms are relatively nonspecific; 50% to 80% of patients with these symptoms do not actually have DVT. Conversely, approximately 80% of patients with symptomatic pulmonary emboli have no signs or symptoms of thrombosis in the lower extremities. Because of the lack of specificity when signs and symptoms are recognized, additional diagnostic tests should be performed to establish the diagnosis of DVT.
Doppler ultrasonography.
B-mode duplex Doppler imaging is currently the most common technique for the diagnosis of symptomatic venous thrombosis, especially when it arises in the proximal lower extremity. With duplex Doppler imaging, the femoral vein can be visualized, and clots may be seen directly. Compression of the vein with the ultrasound probe tip allows assessment of venous collapsibility and the presence of a thrombus diminishes vein wall collapsibility. Abnormal Doppler color flow is an additional sign of DVT. It should be recognized that Doppler imaging does not detect isolated iliac vein or proximal femoral thrombosis. In addition, 2% of patients with initially negative studies will develop DVT in the next 7 days. Repeat testing is warranted on any patient where a high suspicion or risk of DVT is present.
Venography.
Although contrast venography has been the “gold standard” for diagnosis of DVT, other diagnostic studies have nearly equivalent accuracy when performed by a skilled technologist and, in most patients, may replace the need for this study. Venography is moderately uncomfortable, requires the injection of a contrast material that may cause allergic reaction or renal injury, and may result in phlebitis in approximately 5% of patients. However, if noninvasive imaging is normal or inconclusive and the clinician remains concerned given clinical symptoms, venography should be obtained to obtain a definitive answer.
Magnetic resonance venography.
Magnetic resonance venography (MRV) has a sensitivity and specificity comparable to those of venography. In addition, MRV may detect thrombi in pelvic veins that are not imaged by venography. The primary drawback to MRV is the time involved in examining the lower extremity and pelvis and the expense of this technology.
Impedance plethysmography.
Impedance plethysmography measures the change in blood volume in the calf from the impedance of a blood pressure cuff after rapid deflation. The measurements are determined by electrodes placed on the calf. When performed appropriately, it has high sensitivity (91%) and specificity (96%) for proximal vein thromboses. However, patients must lie still for 2 minutes for accurate measurements, and skilled personnel are required for performance of the test. For these reasons, this method is not routinely used.
Treatment of deep vein thrombosis.
The treatment of postoperative DVT requires the immediate institution of anticoagulant therapy. Untreated, symptomatic DVT is associated with a 50% risk of pulmonary embolism, and untreated pulmonary embolism carries a 30% risk of mortality. Treatment may be with either UFH or LMWHs followed by 6 months of oral or subcutaneous anticoagulant therapy. Prolonged anticoagulation (lifetime) is recommended for women who continue to have active cancer (ie, those not in remission after treatment) because they remain at very high risk to rethrombose.
Low-molecular-weight heparin.
Low-molecular-weight heparins (eg, enoxaparin, dalteparin) have been shown to be effective in the treatment of VTE and have a cost-effectiveness advantage over IV heparin in that they may be administered in the outpatient setting. The dosages used in treatment of thromboembolism are unique and weight adjusted according to each LMWH preparation. Because LMWH have a minimal effect on activated PTT, serial laboratory monitoring of PTT levels is not necessary. Similarly, monitoring of anti-Xa activity (except in difficult cases or those with renal impairment) has not been shown to be of significant benefit in a dose adjustment of LMWH. The increased bioavailability associated with LMWH allows for twice-a-day dosing, potentially making outpatient management for a subset of patients an option. A metaanalysis involving more than 1000 patients from 19 trials suggests that LMWH is more effective, safer, and less costly compared with UFH in preventing recurrent thromboembolism.
Unfractionated heparin.
Unfractionated heparin may be the initial treatment of choice in a recent postoperative patient or other clinical settings in which a significant bleeding risk is appreciated and the need for immediate reversal may be evident. An initial bolus is given intravenously followed by a continuous infusion adjusted to therapeutic aPTT levels. Weight-based nomograms to aid in appropriate dosing are widely available and often embedded in hospital pharmacy administration processes.
Long-term anticoagulation.
Traditionally, oral anticoagulation with warfarin was used in the long-term treatment of DVT in cancer patients. Warfarin may be a difficult drug to administer to some patients, however, especially if their nutrition is inadequate, their oral intake is variable, or they require prolonged use of antibiotics or other drugs that might alter the metabolism of warfarin. This is particularly common in women with advanced ovarian cancer. Given the wide variation in the international normalized ratio (INR) in many of these patients who are then predisposed to either bleeding complications or rethrombosis, we have found that it is safer to use subcutaneous LMWH (at therapeutic doses) for prolonged therapy. This clinical experience has been confirmed in a number of large, high-quality, RCTs that have demonstrated the superiority of LMWH over warfarin in the clinical setting. In each study, LMWH resulted in equal or reduced risk of recurrent DVT, with no increase in bleeding events, major or minor. In addition, two trials demonstrated a mortality benefit with LMWH over warfarin. Meta-analyses including these studies have supported these results, including one study with seven RCTs and 1908 cancer patients that demonstrated a hazards ratio of 0.47 (95% confidence interval [CI] 0.3–0.7) for reduction of recurrent DVT. The authors therefore recommend LMWH as the first-line therapy for long-term treatment of DVT in gynecologic cancer patients.
Oral anticoagulants.
If oral anticoagulation must be used (eg, patient refuses daily injections, previous history of HIT), a vitamin K antagonist such as warfarin is preferred over no therapy. The conversion from parenteral heparin or LMWH to oral warfarin may start on the initial day of therapy. The change in INR resulting from warfarin administration often precedes the anticoagulant effect by approximately 2 days, during which time low protein C levels are associated with a transient hypercoagulable state. Therefore, both heparin and warfarin are given, and the heparin is discontinued when the warfarin has reached a therapeutic INR of 2 to 3 for 2 consecutive days. Patients should be cautioned to avoid the use of drugs and dietary products that might alter the metabolism or absorption of warfarin.
New agents such as direct thrombin or factor Xa inhibitors that have been approved for the treatment of acute DVT have not been well tested in cancer populations. Given the significant impact of surgery, chemotherapy, and radiation on a patient’s nutrition, performance status, and comorbid conditions, the authors do not believe there is sufficient data to support the use of the medications over warfarin. Their use is best restricted to scenarios when co-management with a hematologic specialists is available.
Inferior vena cava filter.
In the gynecologic oncology population, acute DVT may often be diagnosed in the immediate pre- or postoperative setting, when pharmacologic anticoagulation is contraindicated because of bleeding risks. In these cases, placement of a retrievable (ie, temporary) inferior vena cava (IVC) filter is an alternative strategy to reducing the risks associated with DVT. The filter can remain in place until bleeding risks decrease, at which time the patient should be started on an appropriate anticoagulation regimen. Filter retrieval is associated with a high technical success rate (85%) and should be pursued given that long-term filter placement is associated with IVC thrombosis, increased recurrent DVT, and the potential for filter migration.
Diagnosis of pulmonary embolism.
Many of the signs and symptoms of pulmonary embolism are associated with other, more commonly occurring pulmonary complications after surgery. The classic findings of pleuritic chest pain, hemoptysis, shortness of breath, tachycardia, and tachypnea should alert the physician to the possibility of a pulmonary embolism. Many times, however, the signs are much more subtle and may be suggested only by a persistent tachycardia or a slight elevation in the respiratory rate. Several diagnostic algorithms have been devised to aid in estimating the likelihood of pulmonary embolism before definitive imaging, such as the Wells or Geneva scoring systems. Interestingly, these algorithms have been compared against physician clinical estimations in a meta-analysis of 52 studies including 55,268 patients. Clinical acumen indeed has comparable sensitivity (0.85 vs. 0.84–0.91) to structured diagnostic algorithms when both are combined with D-dimer testing. Patients with suspected pulmonary embolism can be evaluated initially by chest radiography, electrocardiography, and arterial blood gas assessment. However, a high clinical suspicion or high likelihood based on diagnostic algorithms should prompt immediate spiral CT scan of the chest for definitive diagnosis. Any evidence of abnormality should be further evaluated by spiral CT scan of the chest if renal function permits. Ventilation-perfusion lung scan is an option in the setting of renal insufficiency or failure, although, a high percentage of lung scans may be interpreted as “indeterminate.” In this setting, careful clinical evaluation and judgment are required to decide whether pulmonary arteriography should be obtained to document or exclude the presence of a pulmonary embolism.
The treatment of pulmonary embolism is similar to that of acute DVT in the hemodynamically stable patient. UFH, LMWH, or fondaparinux are all initial parental anticoagulation treatment options. Recommendations from the ACCP currently favor LMWH or fondaparinux for initial treatment, although UFH may be more prudent for the gynecologic oncology patient for whom a perioperative status or high disease burden often confers a bleeding risk requiring easy reversal of anticoagulation method. Hemodynamically unstable patients require immediate care in an intensive care unit (ICU) and assessment for thrombolytic therapy (eg, recombinant tissue type plasminogen activator, streptokinase) or embolectomy if thrombolysis is contraindicated.
Superior vena cava syndrome.
Superior vena cava syndrome is caused by advanced cancers arising in or invading the mediastinum, subsequently obstructing the venous drainage of the head, neck, and upper thoracic regions. Primary tumors are most commonly the cause of this syndrome (bronchogenic carcinomas), although metastasis to the mediastinum from gynecologic cancers can also present in this manner. The vena cava has a low intravascular pressure and is easily compressed by adjacent masses. Most commonly, the symptoms caused by venous obstruction are dramatic swelling and plethora of the head, neck, upper extremities, and chest. Pleural and pericardial effusions can occur with decreased venous return to the heart and a resultant decrease in cardiac output. Patients also commonly complain of a severe headache. A similar clinical syndrome is also seen associated with thrombosis of the subclavian vein and superior vena cava, which is induced by central venous catheters. The diagnosis of the cause of superior vena cava syndrome is critical to selecting proper management. If a localized primary or metastatic neoplasm is identified, immediate radiation therapy is usually the most effective method to achieve resolution. Radiation therapy to the mediastinum in doses of 400 cGy for 3 days and then 150 to 180 cGy per day for a total dose of 3000 to 5000 cGy has been successful in relieving the vascular obstruction. Responses are commonly recognized in 3 to 4 days. Chemotherapy may also play a role, although the resolution of symptoms is usually much slower. Expandable wire stents across the constricted portion of the vena cava have also been used successfully.
In patients in whom thrombosis is the etiology of venous obstruction, immediate anticoagulant therapy should be instituted ( Fig. 16.3 ). The edema and plethora will usually diminish in 1 to 3 days. In many instances, the central venous catheter may be left in place and used. However, if the condition persists or recurs, the catheter should be removed.
Biliary Obstruction
Obstruction of the biliary tree by gynecologic cancers is rare and is usually associated with far advanced cancers and limited life expectancy. Nonetheless, the resulting jaundice and pruritus caused by the obstruction is distressing to the patient and her family. Surgical relief of the obstruction is usually impossible because of the extent of cancer involvement in the region. However, endoscopic placement of a stent in the common duct often will resolve the symptoms and provide a better quality of life. If a stent cannot be placed as a result of extreme compression or other technical reasons, percutaneous placement of a drainage tube into the dilated biliary tree will also resolve symptoms.
Metastatic adenopathy high in the paraaortic chain resulting in biliary obstruction can commonly obstruct the duodenum, leading to gastric outlet obstruction. Although surgical intervention (gastrojejunostomy) or self-expanding metal stents may correct the anatomic problem, careful consideration should be given to the patient’s life expectancy. These are similar considerations to those made in women with small intestinal obstructions and recurrent ovarian cancer discussed previously. In women with just days or weeks to live, placement of a gastrostomy tube may be more prudent.
Treatment-Related Complications
Surgical
Intraoperative and Postoperative Hemorrhage
Intraoperative management of vascular complications.
Surgery for gynecologic cancer often requires extensive dissection in the retroperitoneal space, which may be distorted by cancer metastatic to lymph nodes or invading adjacent structures. It is not surprising, then, that injury to pelvic veins and arteries is common and may result in significant intraoperative blood loss and hemorrhage. Surgeons must be prepared for this eventuality and have in their armamentarium the tools and skills to bring a stop to the bleeding.
Before attempting to bring final control to a significant bleeding area, a few basic principles should be used. First, the patient’s blood volume and coagulation factors must be maintained at all times. Poor communication between the surgeon and the anesthesiologist can lead to significant hypovolemia and cardiovascular instability. Frequent serial laboratory assessments are critical in this setting. Loss of coagulation factors during intraoperative hemorrhage results in continued bleeding that cannot be controlled by surgical means. The surgeon should pack the involved area to allow replacement of blood volume (PRBCs), coagulation factors (FFP, platelets, and cryoprecipitate), and acquisition of appropriate assistance. When the patient is stable and the team is fully prepared, the packed area should be exposed a small area at a time to identify the specific bleeding site.
Before attempting to control the bleeding point, the adjacent anatomy should be identified and protected. In particular, the ureter, adjacent vessels, and viscera must be recognized to avoid further injury. In most cases of arterial bleeding, the artery can be isolated and controlled with sutures. Small arteries may be best controlled by vascular clips, but larger arteries may require sutures with 4-0 or 5-0 vascular suture (Prolene). This holds for injury to the aorta and common and external iliac arteries. Injury to the internal iliac (hypogastric) artery may be controlled with total ligation of the artery. Patency of distal arteries should be confirmed throughout the remainder of the procedure and postoperatively. In rare instances, arterial injuries must be managed by vascular grafting.
Venous bleeding in the pelvis is probably more common given the fragility of the thin vein wall and the extensive network of pelvic venous plexus. Often a specific bleeding point cannot be identified but, after several minutes of direct pressure on the bleeding area, a clot will form over the low-pressure veins, and the bleeding will resolve. If it does not, control will have to be achieved with vascular clips, clamps, and suture ligature. Slow but persistent oozing from unidentified vessels can often be controlled by products that either serve as a matrix for clotting (Avitene, Surgicel, Gelfoam) or supply clotting factors that complete the clotting cascade (CoSeal, TISSEEL, FLOSEAL). After application of any of these products, pressure should be applied to the site for 5 minutes and then the site should be reevaluated. If bleeding has not been controlled, it may be necessary to place a pack and transfer the patient to an ICU. In all cases, it should be emphasized that prompt replacement of clotting factors provided by transfusion of FFP and platelets is critical to achieve hemostasis in the face of hemorrhage.
Replacement of platelets and clotting factors in patients with massive transfusion and microvascular bleeding is dependent on clinical and surgical assessments. Guidelines have been provided by the American Society of Anesthesiologists task force regarding replacement of these products. In general, platelet transfusion is rarely indicated for counts greater than 100,000/µL and usually indicated for counts less than 50,000/µL (with intermediate platelet counts 50,000/µL to 100,000/µL, transfusion should be based on the risk of bleeding). FFP therapy is indicated in massively transfused patients with microvascular bleeding or hemorrhage if the prothrombin time (PT) or aPTT values exceed 1.5 times the normal values. In cases of massive intraoperative blood loss, it may be prudent to administer FFP empirically. This strategy is aimed at preventing the patient from becoming hypocoagulable while awaiting the laboratory results (PT and PTT), which may take nearly an hour and another hour to thaw the FFP before it is available to be administered. Cryoprecipitate transfusions are recommended for correction of microvascular bleeding in massively transfused patients with fibrinogen concentrations less than 80 to 100 mg/dL. For trauma patients with massive blood loss, on the order of 30% to 40% of their blood volume, a transfusion ratio of 1 : 1 : 1 for PRBCs, FFP, and platelets has been consistently reported to be associated with decrease in death caused by hemorrhage. If surgical bleeding has taken on this level of severity, such a massive transfusion protocol should be initiated.
Bleeding from the sacrum is usually not encountered except in the course of performing a total pelvic exenteration or rectosigmoid resection or during a sacral-colpopexy. If bleeding from the sacrum cannot be controlled by suture ligatures or vascular clips, placement of sterile thumbtacks pushed into the sacral bone will usually compress veins exiting the sacrum and achieve hemostasis.
Hypogastric (internal iliac) artery ligation.
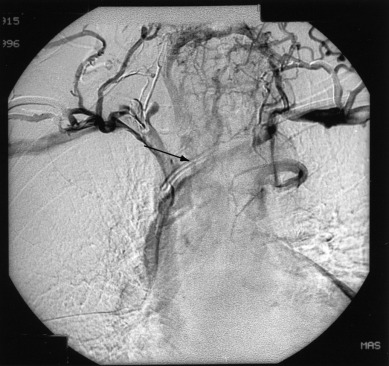
When the usual steps to control hemorrhage have failed, hypogastric artery ligation should be performed. Although the arterial blood supply to the pelvis is rich with anastomosis, ligation of the hypogastric arteries will usually decrease the arterial and venous pressure to the point at which (in a patient who is not hypocoagulable) venous bleeding will slow and can either be controlled by ligature or will clot with prolonged packing.
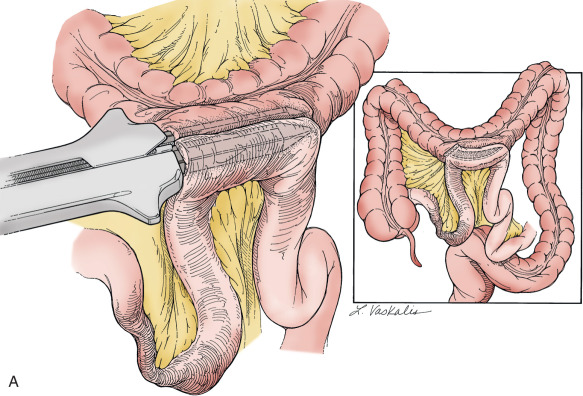
To safely perform hypogastric artery ligation, the vascular anatomy must be exposed, and adjacent structures, especially the ureter, must be identified. A retroperitoneal approach should be taken. The peritoneum overlying the psoas muscle (lateral to the external iliac artery) should be incised parallel to the artery. As the peritoneum is mobilized medially, the external iliac artery will first be identified. Dissection cephalad along the external iliac artery will identify the common iliac artery and the bifurcation of the internal iliac artery. Invariably, the ureter crosses the pelvic brim at the bifurcation of the common iliac artery. At this location, the ureter will be identified attached to the medial peritoneum. Further opening of the retroperitoneal space keeping the iliac vessels lateral and the ureter medial will create the pararectal space and further expose the internal iliac artery. Be aware that the common external and internal iliac veins lie just beneath their respective arteries. Without clear identification of these veins, injury can occur, which will further complicate the procedure. The hypogastric artery bifurcates into an anterior and posterior branch 2 to 3 cm from its origin from the common iliac artery. Because most bleeding arises from the blood supply from the anterior division, the anterior branch should be ligated if at all possible. (Ligation of the posterior branch increases the risk of buttock pain and potential necrosis of the gluteus.) A right angle clamp should be carefully passed from lateral to medial beneath the hypogastric artery. A heavy suture (eg, 0-silk) should be used to ligate the artery ( Fig. 16.4 ). There is no reason to transect the artery between two ligatures. It is usually best to perform bilateral hypogastric artery ligation as the collateral blood supply crosses over the midline.
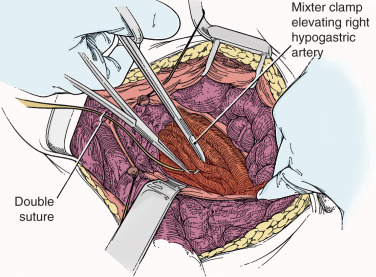
Finally, if all methods to control hemorrhage have been unsuccessful, the bleeding site should be packed firmly, and the abdomen should be closed. The patient should be taken to the surgical ICU and stabilized. Central monitoring and blood product replacement should be the primary focus of management. After the patient is stabilized, attempts at angiographic embolization should be considered. Ultimately, after 24 to 48 hours, the patient should be returned to the operating room and reexplored and the pack removed carefully. Surprisingly, many times the bleeding will have stopped as a result of compression of the injured veins and correction of the hypocoagulable state.
Management of shock.
During and after gynecologic surgery, blood-volume deficit that results from intraoperative blood loss or postoperative hemorrhage is the most common cause of shock. This shock usually is manifested by arterial hypotension, tachycardia, a weak pulse, anxiety, skin pallor, diminished urinary output, and peripheral vasoconstriction. In addition to hemorrhage (hypovolemic shock), the differential diagnosis of shock must include many other causes, such as cardiogenic (myocardial infarction) and cardiac compressive conditions (cardiac tamponade or pneumothorax), sepsis, drug overdose, and pulmonary embolism. Appropriate studies are dictated by the patient’s signs and symptoms.
Consideration should be given to obtaining arterial blood gas analysis, an electrocardiogram, a chest radiograph, blood chemistry studies, and blood cultures. The patient should be prepared for blood transfusion. The degree and duration of postoperative shock determine the need for resuscitation, central venous pressure monitoring, and Swan-Ganz pulmonary artery catheterization.
Resuscitation of a hemorrhaging patient during or after gynecologic surgery involves stabilization of the hemodynamic status and correction of the cause of the blood loss. When the hemorrhage is massive, fluid, electrolyte, and hemodynamic shifts are likewise massive. Central to stabilization efforts are the replacement and maintenance of adequate intravascular volume.
Central monitoring.
Invasive cardiovascular monitoring may be lifesaving for patients with massive hemorrhage or patients who are at additional risk because of preexisting cardiopulmonary disorders. The monitoring allows the rational use of fluids and cardioactive medications while avoiding their complications.
In patients with marked hemodynamic instability, peripheral artery cannulation allows continuous monitoring of systemic arterial pressure and ready access to obtain repeated analysis of arterial blood gases. The radial artery is usually chosen because of accessibility and has good collateral circulation, although the brachial and femoral arteries may be used. The complications of arterial cannulation include catheter-related septicemia (4% in one large study), local infection (as high as 18%), and arterial embolization (0.2%–0.6%).
In patients without cardiopulmonary disease, monitoring of central venous pressure along with monitoring of the vital signs, urine output, and other clinical signs usually provide sufficient information for appropriate fluid resuscitation. In addition, central venous pressure monitoring avoids several of the complications of a pulmonary artery (Swan-Ganz) catheter.
A central venous catheter may be introduced into the great intrathoracic veins by way of the antecubital, external or internal jugular, or subclavian veins. Cannulation of the right internal jugular vein, which provides a straight course to the right atrium, has the lowest overall complication rate. In all cases, a chest radiograph should be obtained immediately after catheter insertion to confirm proper location and to assess for possible pneumothorax.
The usefulness of the Swan-Ganz catheter in critically ill patients (even those without heart disease) who do not respond to therapy based on an initial noninvasive assessment is primarily limited to its use as a diagnostic monitoring tool. Several randomized trials, including those of high-risk surgical patients, have confirmed that there is no mortality benefit to use of the Swan-Ganz catheter. It does provide additional diagnostic information concerning unsuspected cardiac dysfunction, pulmonary artery embolization, or sepsis. Patients without primary myocardial insult but with hypotension and evidence of inadequate perfusion of vital organs (eg, oliguria, acidosis, mental obtundation) can be managed with more information ( Table 16.2 ).
In patients with cardiac or pulmonary disease, cardiac output and resistance measurements allow the proper use of pressors, afterload and preload reducers, and fluids. In addition, if sepsis is part of the clinical picture, careful monitoring of pulmonary capillary wedge pressures may be necessary to prevent pulmonary edema, which is seen with even mild increases in left atrial pressures as a result of the increased permeability of the pulmonary vascular bed. This increased permeability also may be seen in patients in hypovolemic shock, again leading to pulmonary edema at relatively normal wedge pressures. Finally, invasive monitoring not only provides a direct measurement of cardiac function but also provides information within minutes about the effects of therapy.
These catheters may be placed from the antecubital fossa, although the percutaneous subclavian or internal or external jugular vein approaches are more commonly used. Complications of pulmonary artery catheters include pulmonary infarction distal to the catheter (1%–2% of cases), pulmonary artery rupture (0.2% of cases), balloon rupture (3% of cases), and sepsis (2% of cases), all of which are made more likely by prolonged use of the catheter.
Intraoperative Genitourinary Injuries
Given the close anatomic relationships of the gynecologic organs and the urinary tract, ureteral and bladder injury are to be anticipated, even in the hands of the most skilled surgeon. Prevention of urinary tract injury is predicated on the identification of the key urinary tract structures before embarking on radical or extended surgery for gynecologic cancers. Retroperitoneal exploration by opening the lateral retroperitoneal spaces (pararectal and paravesical spaces) allows for identification of the ureter and lateral bladder (and key vascular structures). If the medial pelvic peritoneum requires resection, the ureter must be detached from the peritoneum and mobilized laterally (ureterolysis). The ureter may be dissected throughout its entire pelvic course to the bladder, although between the uterine artery crossing the ureter and the insertion of the ureter into the bladder, techniques similar to those required for a type II or III radical hysterectomy will need to be used. Identification of the bladder is usually not a problem; however, because of anatomic distortion by advanced cancer, radiation therapy, or extensive adhesions from prior surgery, the bladder sometimes is not easily recognized. One simple method to identify the bladder is to fill it retrograde through the indwelling Foley catheter. With the bladder distended, dissection of the uterus or tumor from the bladder may be facilitated. Opening the retropubic space (space of Retzius) and the paravesical space also facilitates identification and protection of the bladder.
Injury to the bladder is usually easily corrected at the time of surgery. Incisions in the dome of the bladder should be closed in two layers with 3-0 or 2-0 delayed absorbable suture. The bladder should be allowed to heal by leaving the Foley catheter to dependent drainage for approximately 5 days. Cystotomies at the base of the bladder have a higher risk of fistula formation and ureteral occlusion. Furthermore, they may be more difficult to recognize. Whenever there is concern about potential bladder injury, the bladder should be filled in a retrograde fashion with either sterile infant formula or indigo carmine–dyed saline. The authors prefer infant formula because the formula does not stain tissues and potentially obscure the site of injury. After the cystotomy in the base of the bladder is identified, it is important to assess the location of the ureteral orifices. This may be accomplished by cystoscopy or by opening the dome of the bladder and directly visualizing the orifices. The administration of IV indigo carmine may aid in the identification of the orifices. Closure of the cystotomy should again be accomplished using two layers of delayed absorbable suture. If the cystotomy is near the ureteral orifice, retrograde placement of a stent is prudent to ensure that there is no occlusion or narrowing of the ureter. If the pelvis has been previously radiated, the authors recommend placement of an omental J-flap between the bladder and the vaginal cuff to bring a new, nonirradiated blood supply into this area. The Foley catheter should be left to drain for several weeks, and a cystogram should be obtained before the decision to remove the catheter.
Injury to the ureter as it passes through the pelvis may be managed by several methods depending on the location and extent of the injury. Ureteral injury above the pelvic brim is usually best managed by end-to-end anastomosis over a ureteral stent ( Fig. 16.5 ). Injury below the pelvic brim may be best corrected by a ureteroneocystostomy with psoas hitch or a Boari flap ( Fig. 16.6 ). In either method of repair placement, a closed suction drain in the pelvis is advised to prevent a urinoma from a leak of the anastomosis.


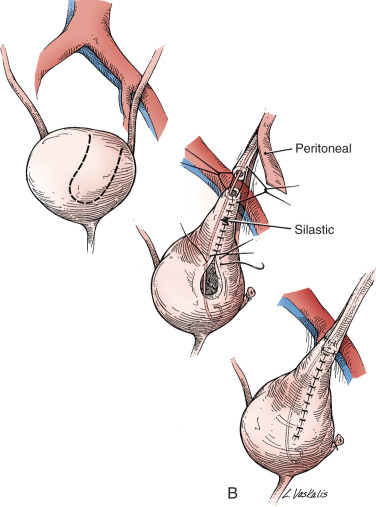
In cases of suspected injury to the ureter, IV indigo carmine should be injected, and the pelvis should be inspected for spill of dye-colored urine. If the extent of ureteral injury is a clamp crush or ligature, placement of a ureteral double-J stent left in place for 6 weeks will usually allow the injured ureter to recover and at the same time prevent ureteral stricture.
Postoperative Urinary Tract Injury
Unless bilateral ureteral obstruction has been caused by surgery, most patients with postoperative anuria or severe oliguria will have these findings secondary to prerenal hypovolemia, which will resolve with hydration and diuresis. However, unilateral ureteral injury may not be recognized until several days postoperatively, manifest by flank pain, pyelonephritis, or a slight rise in serum creatinine. The volume of urinary output is rarely altered. When postoperative ureteral obstruction is suspected, evaluation may include intravenous pyelography (IVP) or CT scan with contrast (when the serum creatinine level is normal) or with a renal ultrasound or Lasix renal scan. If ureteral obstruction is discovered, initial management should include cystoscopy with retrograde stent placement. If successful, the obstruction is likely a result of tethering from nearby sutures or extrinsic compression from a mass (hematoma, lymphocyst, tumor). Leaving the stent in place for 6 weeks and then reevaluating with follow-up IVP is recommended. If a stent cannot be placed, however, consideration should be given to reexploration to correct the obstruction. If reoperation is not reasonable, a PCN tube should be placed.
Urinary tract fistulae are recognized complications of radical hysterectomy for the primary treatment of cervical cancer, with recent estimates at just over 1% incidence. It is thought that the extensive dissection of the distal ureter and base of the bladder may lead to ischemic necrosis and the development of a vesicovaginal or ureterovaginal fistula. In contemporary reports, these fistulas occur in only 1% to 3% of cases. The risk of fistula is increased when surgery is performed after prior pelvic radiation therapy.
Vaginal leakage of fluid during the first 10 weeks postoperatively is an ominous finding and requires evaluation for a urinary tract fistula. Confirming the presence of a fistula and, if present, identifying the location are the next priorities. Clinic-based examinations include retrograde filling of the bladder with dyed (eg, indigo carmine) saline and inspection of the vaginal vault for vesicovaginal fistula and administration of IV indigo carmine or oral pyridium with vaginal inspection for ureterovaginal fistula. Both of these methods do carry false-negative risks, and even if negative, in cases of continued vaginal leakage, a definitive CT scan with IV contrast (CT urogram or CT of the abdomen and pelvis with delayed phase contrast) should be obtained. Acute vesicovaginal fistula should be managed by decompression of the bladder by placement of an indwelling Foley catheter to allow continuous drainage. This management often allows the fistula to close spontaneously. If a ureterovaginal fistula is discovered, a ureteral stent should be placed across the section of ureter that is fistualized. The stent may be placed retrograde at the time of cystoscopy or antegrade via a PCN. This usually allows the ureteral injury to heal “over” the stent. Throughout attempts at conservative management, prevention of UTI is an important priority. If after 6 weeks of conservative management the vesicovaginal or ureteral fistula has not resolved, surgical correction will be required.
Bladder Dysfunction After Radical Surgery
Voiding dysfunction after radical hysterectomy is commonly recognized and should be discussed in the preoperative informed consent. The exact frequency of occurrence depends on how the problem is diagnosed (patient report vs. prospective urodynamic testing) but occurs to a greater or lesser degree in nearly all patients. It is clear that a less radical hysterectomy (type I or II) is associated with a significantly lower incidence of bladder dysfunction as compared with the traditional radical (type III) hysterectomy.
Serial cystometric studies of patients undergoing radical hysterectomy have defined the natural history of bladder function in the perioperative period. There is a nearly uniform development of detrusor hypertonia characterized by low capacity, high resting tone, and high filling pressure in the immediate postoperative phase. Bladder insensitivity to filling is also present. The patient often has difficulty initiating her stream and may have overflow incontinence when the capacity is exceeded. Hypertonia generally subsides within 3 to 6 months, but other abnormalities often persist for years. The duration of bladder dysfunction is also variable, with extremes recognized as full recovery of bladder function to the rare patient who will require lifetime intermittent self-catheterization to achieve adequate bladder drainage. Many patients have persistent decrease in bladder sensation or prolonged urinary hesitancy.
The pathophysiologic mechanism of voiding dysfunction after radical hysterectomy is still not clearly understood. Some investigators have proposed that incomplete innervation of the bladder produced a temporary parasympathomimetic predominance that usually resolves with nerve regeneration. The use of parasympatholytic drugs, however, has been ineffective in altering the detrusor muscle function in this circumstance. Forney and colleagues have suggested that disruption of the sympathetic fibers that travel through the paracervical web results in loss of inhibition for the detrusor and trigone, leaving an uncoordinated parasympathetic dominance. This is supported by the observation that incomplete division of the cardinal ligament results in decreased postoperative detrusor hypertonia compared with complete division.
It also seems clear that overdistention of the bladder aggravates bladder dysfunction. Therefore, a variety of techniques have been proposed to avoid overdistention, including short- or long-term use of a urethral Foley catheter, suprapubic catheter, or intermittent self-catheterization. All techniques have their proponents and varying degrees of success, but none has been proven to be superior.
Intraoperative Gastrointestinal Injuries
A mechanical bowel preparation with preoperative oral antibiotics (eg, erythromycin and neomycin) should be planned for all patients undergoing major abdominal surgery for which bowel surgery is anticipated. Several retrospective and prospective cohort studies of patients undergoing colorectal resection for colon cancer consistently demonstrate decreases in surgical site infection, anastomotic leak, and ileus in patients receiving both mechanical and antibiotic preparation. In a prospective cohort study of over 1500 patients, mechanical preparation plus oral antibiotics resulted in lower infection (4.6% vs. 12.4%; P <0.001) and lower rate of ileus (3.8% vs. 8.9%; P = 0.006) compared with mechanical preparation alone. Additionally, researchers using a national surgical quality database of more than 8000 patients found that surgical site infection (6.1% vs. 12.0% vs. 14.3%), anastomotic leak (2.0% vs. 3.6% vs. 4.5%), postoperative ileus (9.1% vs. 12.1% vs. 15.1%), and 30-day mortality rate (0.4% vs. 0.6% vs. 1.5%) were all reduced in the dual prep method versus mechanical preparation alone versus no preparation. It should be noted that all patients in these studies also received parenteral antibiotics, confirming the additional benefit of the dual preparation. Bowel preparation may also reduce the risk of intraoperative intestinal injury, and if injury does occur, spill of GI contents can be minimized. Mechanical methods to prepare the bowel include the use of cathartics such as magnesium citrate or the ingestion of 4 L of polyethylene glycol (GoLYTELY).
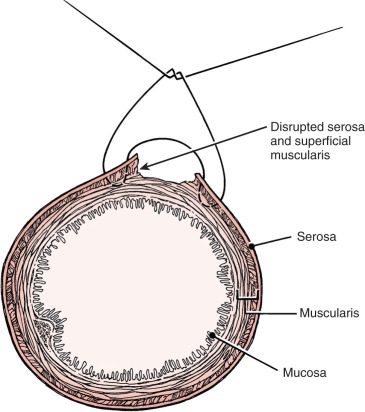
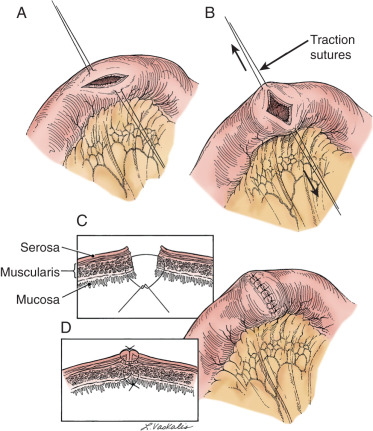
Prior abdominal surgery and radiation therapy increase the probability of intraoperative injury to the small intestine. Adhesions to the anterior abdominal wall or small intestines adherent to the pelvic peritoneum increase the risk of entry into the intestines as adhesions are lysed. Interruption of the serosal and superficial muscular layers should be repaired with interrupted 2-0 or 3-0 sutures. Care should be taken to avoid narrowing of the intestinal caliber ( Fig. 16.7 ). If the entire thickness of the intestine is entered, the segment of bowel must be assessed to decide whether primary closure should be undertaken or whether bowel resection and anastomosis should be performed. Primary closure is usually safely accomplished if the closure can reapproximate well-perfused bowel under no tension on the suture line and the bowel lumen is not narrowed. If these conditions cannot be met, resection and anastomosis will be necessary.
Primary closure of an enterotomy should be in two layers, the first being an interrupted layer of 2-0 or 3-0 polyglycolic acid suture incorporating the mucosa and muscularis, with the knot tied into the intestinal lumen. A second imbricating layer may be of either absorbable or nonabsorbable suture and incorporates the serosa and superficial muscularis ( Fig. 16.8 ). Attention to the direction of closure of the enterotomy should ensure that the lumen is not narrowed.
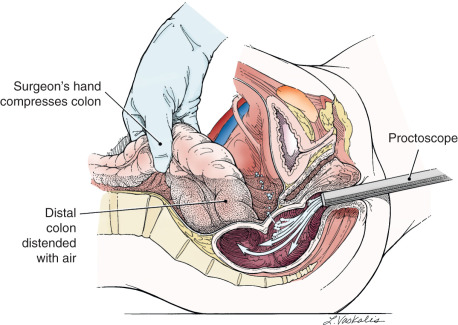
Injury to the colon most commonly occurs in the pelvis, and the risk is increased in the face of prior pelvic surgery, cancer involving the colon, or prior radiation therapy. If colonic injury occurs, the decision must be made as to whether a primary closure is sufficiently safe or whether the fecal stream should be temporarily diverted by performing a transverse loop colostomy or ileostomy. In most cases, primary closure without diversion may be performed; especially if the patient has had a mechanical bowel preparation. The principles for closure require viable tissue and no tension on the closure. In patients who have had prior pelvic radiation, the risk of perforation of the colostomy closure is significantly increased, and diversion is usually advised. After a two-layered closure of a rectosigmoid injury, the authors routinely perform proctoscopy and a “bubble test” ( Fig. 16.9 ). If either test suggests a defect in the reanastomosis, a diverting ostomy is again recommended.
Postoperative Gastrointestinal Complications
Ileus.
After abdominal or pelvic surgery, most patients experience some degree of intestinal ileus. The exact mechanism by which this arrest and disorganization of GI motility occurs is unknown, but it appears to be associated with the opening of the peritoneal cavity and is aggravated by manipulation of the intestinal tract and prolonged surgical procedures. (Ileus is much less commonly associated with minimally invasive surgical procedures. If ileus were to occur after laparoscopy or robotic surgery, the surgeon should strongly consider a more serious bowel injury and evaluate the patient accordingly.) Infection, peritonitis, postoperative hypervolemia causing bowel wall edema, and electrolyte disturbances may also result in ileus. For most patients undergoing gynecologic cancer operations, the degree of ileus is minimal and GI function returns relatively rapidly, allowing the resumption of oral intake within a few days of surgery. Patients who have persistently diminished bowel sounds, abdominal distention, and nausea and vomiting require further evaluation and more aggressive management.
Ileus is usually manifest by abdominal distention and should be evaluated initially by physical examination assessing the quality of bowel sounds and searching for tenderness or rebound on palpation. The possibility that the patient’s signs and symptoms may be associated with a more serious intestinal obstruction or other intestinal complication must be considered. Pelvic examination should be performed to evaluate the possibility of a pelvic abscess or hematoma that may contribute to the ileus. Abdominal radiography to evaluate the abdomen in the supine position and in the upright position usually will aid in the diagnosis of an ileus. The most common radiographic findings include dilated loops of small and large bowel and air–fluid levels in the upright position. In postoperative patients, especially in the upright position, the flat plate of the abdomen may also show evidence of free air. This is a common finding after surgery; it lasts 7 to 10 days in some instances and is not indicative of a perforated viscus in most patients. The remote possibility of distal colonic obstruction or pseudoobstruction (Ogilvie syndrome) suggested by a dilated cecum should be excluded by rectal examination, proctosigmoidoscopy, or barium enema.
The initial management of a postoperative ileus is aimed at GI tract decompression and maintenance of appropriate IV replacement fluids and electrolytes.
- 1.
If a patient has moderate to severe vomiting, not improved with bowel rest and antiemetics, an NG tube should be placed to evacuate the stomach of its fluid and gaseous contents. Prolonged NG suction continues to remove swallowed air, which is the most common source of air in the small bowel.
- 2.
Fluid and electrolyte replacement must be adequate to keep the patient well perfused. Significant amounts of third-space fluid loss occur in the bowel wall, the bowel lumen, and the peritoneal cavity during the acute episode. GI fluid losses from the stomach may also lead to a metabolic alkalosis and depletion of other electrolytes. Careful monitoring of serum chemistries and appropriate replacement are necessary.
- 3.
Most cases of severe ileus begin to improve over a period of several days. In general, this is recognized by reduction in the abdominal distention, return of normal bowel sounds, and passage of flatus or stool. Follow-up abdominal radiographs should be obtained as necessary for further monitoring.
- •
When the GI tract function appears to have returned to normal, the NG tube may be removed, and a liquid diet may be instituted.
- •
If a patient shows no evidence of improvement during the first 48 to 72 hours of medical management, other causes of ileus should be sought. Such cases may include ureteral injury, peritonitis from pelvic infection, unrecognized GI tract injury with peritoneal spill, or fluid and electrolyte abnormalities such as hypokalemia. In the evaluation of persistent ileus, the use of water-soluble upper GI contrast studies may assist in the resolution of the ileus, the recognition of a small bowel obstruction, or injury (leak) of the GI tract.
- •
Small bowel obstruction.
Obstruction of the small bowel after abdominal surgery occurs in approximately 1% to 2% of patients, but it may be more common after radical gynecologic oncology procedures as a result of the extensive dissection and manipulation of the small bowel. (Again, this complication is less common in patients who have undergone minimally invasive surgery.) The most common cause of small bowel obstruction is adhesions to the operative site. If the small bowel becomes adherent in a twisted position, partial or complete obstruction may result from distention, ileus, or bowel wall edema. Less common causes of postoperative small bowel obstruction include entrapment of the small bowel into an incisional hernia and an unrecognized defect in the small bowel or large bowel mesentery. Early in its clinical course, a postoperative small bowel obstruction may exhibit signs and symptoms identical to those of ileus. Initial conservative management as outlined for the treatment of ileus is appropriate. Because of the potential for mesenteric vascular occlusion and resulting ischemia or perforation, worsening symptoms of abdominal pain, progressive distention, fever, leukocytosis, or acidosis should be evaluated carefully because immediate surgery may be required.
However, in most cases of postoperative small bowel obstruction, the obstruction is only partial, and the problem usually resolves with conservative management.
- •
After several days of conservative management (NG tube drainage, fluid and electrolyte replacement), further investigation may be necessary. Evaluation of the GI tract with barium enema and an upper GI series with small bowel follow-through is appropriate. Alternatively, an abdominal and pelvic CT scan with GI contrast may be useful in identifying the location of obstruction (“transition point”) and evaluates for the presence of an abscess, lymphocele, or ureteral injury. In most cases, complete obstruction is not documented, although a narrowing or tethering of the segment of small bowel may indicate the site of the problem.
- •
Further conservative management with NG decompression and IV fluid replacement may allow time for bowel wall edema or torsion of the mesentery to resolve.
- •
If resolution is prolonged and the patient’s nutritional status is marginal, the use of TPN may be necessary.
- •
- 4.
Conservative medical management of postoperative small bowel obstruction usually results in complete resolution. However, if persistent evidence of small bowel obstruction remains after full evaluation and an adequate trial of medical management, exploratory laparotomy may be necessary to surgically evaluate and manage the obstruction. In most cases, lysis of adhesions is all that is required, although a segment of small bowel that is badly damaged or extensively sclerosed from adhesions may require resection and reanastomosis.
Colonic obstruction.
Postoperative colonic obstruction after gynecologic oncology surgery is exceedingly rare. Advanced ovarian carcinoma is the most common cause of colonic obstruction in postoperative gynecologic surgery patients and is caused by extrinsic impingement on the colon by the pelvic malignancy. Occult intrinsic colonic lesions (eg, colon cancer) also may cause obstruction. When colonic obstruction is manifest by abdominal distention and abdominal radiographs reveal a dilated colon and enlarging cecum, further evaluation of the large bowel is required by barium enema or colonoscopy. Dilation of the cecum to more than 10 cm to 12 cm in diameter as viewed by abdominal radiography requires immediate evaluation because there is a higher probability of colonic perforation. When there is no logical cause for a mechanical obstruction, Ogilvie syndrome should be considered. Colonoscopy should be performed, and the colonic gas should be evacuated. If a true mechanical obstruction is documented, surgical decompression by performing a colectomy, colostomy, or cecostomy may be required on an urgent basis. In some circumstances, an intraluminal stent may be placed endoscopically. Surgery should be performed as soon as the obstruction is documented. Conservative management of colonic obstruction is not appropriate because the complication of colonic perforation has an exceedingly high mortality rate.
Diarrhea.
Episodes of diarrhea often occur after abdominal and pelvic surgery as the GI tract returns to its normal function and motility. However, prolonged and multiple episodes may represent a pathologic process such as impending small bowel obstruction, colonic obstruction, or pseudomembranous colitis. Excessive amounts of diarrhea should be evaluated by abdominal radiographs and stool samples tested for the presence of ova and parasites, bacterial culture, and Clostridium difficile toxin. Proctoscopy and colonoscopy may also be advisable in severe cases. Evidence of intestinal obstruction should be managed as outlined previously. Infectious causes of diarrhea should be managed with the appropriate antibiotics and fluid and electrolyte replacement. C. difficile –associated pseudomembranous colitis may result from recent exposure to any antibiotic. Discontinuation of these antibiotics (unless they are needed for another severe infection) is advisable, along with the institution of appropriate therapy. Because of the expense of vancomycin, the authors usually institute therapy with oral metronidazole for mild to moderate cases. Because of rising resistance to oral metronidazole, initiation of treatment with oral vancomycin may be necessary because clinical success (resolution of diarrhea) is lower (73% vs. 81%; P = 0.02) with metronidazole. Therapy should be continued until the diarrhea abates, and several weeks of oral therapy may be required to obtain complete resolution of the pseudomembranous colitis.
Fistula.
Gastrointestinal fistulas are relatively rare after gynecologic surgery. They are most often associated with malignancy, prior radiation therapy, or surgical injury to the large or small bowel that was unrecognized or a leak from an anastomosis. Signs and symptoms of GI fistula are often similar to those of small bowel obstruction or ileus except that a fever is usually a more prominent component of the patient’s symptoms. When fever is associated with GI dysfunction postoperatively, evaluation should include early assessment of the GI tract for its continuity. When fistula is suspected, the use of water-soluble GI contrast material is advised to avoid the complication of barium peritonitis. Evaluation with abdominal pelvic CT scan may also assist in identification of a fistula and associated abscess. Recognition of an intraperitoneal GI leak or fistula formation usually requires immediate surgery unless the fistula has drained spontaneously through the abdominal wall or vaginal cuff.
An enterocutaneous fistula arising from the small bowel and draining spontaneously through the abdominal incision may be managed successfully with medical therapy. Therapy should include NG decompression, replacement of IV fluids, TPN, and appropriate antibiotics to treat an associated mixed bacterial infection. If the infection is under control and there are no other signs of peritonitis, the surgeon may consider allowing potential resolution of the fistula over a period of up to 6 weeks. Some authors have suggested the use of somatostatin to decrease intestinal tract secretion and allow earlier healing of the fistula. In many cases, especially if the bowel was not radiated, the fistula will close spontaneously with this mode of management. If the enterocutaneous fistula does not close with conservative medical management, surgical correction with resection, bypass, or reanastomosis will be necessary in most cases.
A rectovaginal fistula that occurs after gynecologic surgery is usually the result of surgical trauma that may have been aggravated by the presence of extensive adhesions or tumor involvement of the rectosigmoid colon and cul de sac. Low rectal anastomoses are also at risk to breakdown and lead to a rectovaginal fistula. Perforation of a colonic diverticulum is another possible cause for a colovaginal fistula. A small rectovaginal fistula may be managed with a conservative medical approach in the hope that decreasing the fecal stream will allow closure of the fistula. A small fistula that allows continence except for an occasional leak of flatus may be managed conservatively until the inflammatory process in the pelvis resolves. At that point, usually several months later, correction of the fistula is appropriate. Larger rectovaginal fistulas that have no hope of closing spontaneously are best managed by performing an initial diverting colostomy followed by repair of the fistula after inflammation has resolved. After the fistula closure is healed and deemed successful, the colostomy may be taken down and closed.
Lymphedema
Lower extremity lymphedema can occur after pelvic, paraaortic, or inguinal lymph node dissections that are commonly part of comprehensive surgical staging for gynecologic cancers. In addition to swelling, patients may report sensations of heaviness, numbness, aching, and pain. When defined as early accumulation of fluid that resides with limb elevation, the rate of lymphedema has been reported as high as 35%, although symptomatic rate of occurrence may be lower (5%–15%). This discrepancy is secondary to both a lack of a consensus definition of lymphedema and a paucity of studies. Patients presenting with lymphedema postoperatively should have early referral to physical therapy services specializing in lymphedema to reduce long-term swelling and improve quality of life.
Lymphocysts
The occurrence of lymphocysts after pelvic and paraaortic lymphadenectomy occurs in approximately 20% of cases, but it symptomatic in as few as 2% to 6%. It has been suggested that prevention requires the ligation of the ascending lymphatics at the time of lymphadenectomy. Furthermore, there is evidence that the use of low-dose heparin increases lymphatic drainage and lymphocyst formation. In years past, the parietal peritoneum was closed after lymphadenectomy, and a closed-suction drain was placed in the retroperitoneal space to aspirate any lymphatic fluid. Certainly, a suction drain is reasonable and likely does prevent lymphocysts whenever the retroperitoneum is closed. Currently, most gynecologic oncologists do not close the peritoneum, allowing the lymphatic fluid to drain into the peritoneal cavity and be reabsorbed. Retrospective reports indicate that there is no increase in lymphocyst formation using this strategy. However, the problem of lymphocyst formation has not been entirely eliminated.
Lymphocysts may be recognized clinically by palpation of a mass along the pelvic sidewall or in the iliac fossa on abdominal examination. Most will be asymptomatic. However, when a lymphocyst is found, investigation should be undertaken to evaluate for the possibility of ureteral or venous obstruction. If hydroureter is found, the lymphocyst should be drained. This may usually be accomplished by an interventional radiologist who can place a percutaneous drain in the lymphocyst. Suction drainage will collapse the lymphocyst and allow the cyst walls to sclerose together. If percutaneous drainage is not feasible or is unsuccessful, surgical exploration may be required to excise a wall of the lymphocyst and allow the lymphatic fluid to drain into the peritoneal cavity. There are reports of successfully performing this surgery laparoscopically. Placing an omental J flap into the cavity of the lymphocyst has been suggested to provide additional routes of lymphatic drainage. When extrinsic venous compression is discovered to be associated with a lymphocyst, the patient should be evaluated for the possibility of a venous thrombosis. If a venous thrombosis is found, the authors advise anticoagulation in order to stop propagation and allow stabilization of the thrombus. Thereafter the lymphocyst should be drained. Drainage of the lymphocyst before anticoagulation may result in venous decompression and subsequent embolization of the thrombus. Rarely, lymphocysts will become infected and require drainage and antibiotic therapy.
Lymphocysts can also occur in the inguinal region after inguinofemoral lymphadenectomy performed as part of the staging and treatment of vulvar carcinoma. Small, asymptomatic lymphocysts may be managed by observation. However, larger lymphocysts usually cause pain or lymphedema and require drainage with placement of a closed-suction drain. If repeated aspiration is required, sclerotherapy should be considered.
Postoperative Infections
Infections are a major source of morbidity in the postoperative period. Risk factors for infectious morbidity in gynecologic oncology patients include a lack of perioperative antibiotic prophylaxis, contamination of the surgical field from infected tissues or from spillage of large bowel contents, an immunocompromised host, diabetes, poor nutrition, chronic and debilitating severe illness, poor surgical technique, and preexisting focal or systemic infection. Sources of postoperative infection can include the lung, urinary tract, surgical site, pelvic sidewall, vaginal cuff, abdominal wound, and sites of indwelling IV catheters. Early identification and treatment of any infections will result in the best outcome from these potentially serious complications.
Although infectious morbidity is an inevitable complication of surgery, the incidence of infections can be decreased by the appropriate use of simple preventive measures. In cases that involve transection of the large bowel, spillage of fecal contents also inevitably occurs. Preoperative mechanical preparation in combination with oral and systemic antibiotic prophylaxis will help decrease the incidence of postoperative pelvic and abdominal infections in these patients. The surgeon can further decrease the risk of postoperative infections by using meticulous surgical technique. Blood and necrotic tissue are excellent media for the growth of aerobic and anaerobic organisms. When there is higher-than-usual potential for serum and blood to collect in spaces that have been contaminated by bacterial spill, closed-suction drainage may reduce the risk of infection. Elective surgical procedures should be postponed in patients who have an infection preoperatively.
Historically, the standard definition of febrile morbidity for surgical patients has been the presence of a temperature of 100.4°F (38°C) or higher on two occasions at least 4 hours apart in the postoperative period, excluding the first 24 hours. However, other sources have defined fever as two consecutive temperature elevations greater than 101.0°F (38.3°C). Febrile morbidity has been estimated to occur in as many as half of patients; however, it is often self-limited, resolves without therapy, and is usually noninfectious in origin.
The assessment of febrile surgical patients should include a review of the patient’s history with regard to risk factors. Both the history and the physical examination should focus on the potential sites of infection. The examination should include inspection of the pharynx, a thorough pulmonary examination, percussion of the kidneys to assess for costovertebral angle tenderness, inspection and palpation of the abdominal incision, examination of sites of IV catheters, and an examination of the extremities for evidence of DVT or thrombophlebitis. In gynecologic surgery patients, an appropriate workup may also include inspection and palpation of the vaginal cuff for signs of induration, tenderness, or purulent drainage. A pelvic examination should also be performed to identify a mass suggesting a pelvic hematoma or abscess and to recognize signs of pelvic cellulitis.
Patients with a fever in the early postoperative period should have an aggressive pulmonary toilet, including incentive spirometry. If the fever persists beyond 72 hours postoperatively, additional laboratory and radiologic data may be obtained. The evaluation may include complete and differential white blood cell counts and a urinalysis. A routine urine culture has a yield of only 9%; therefore, one should not be sent unless indicated by the urinalysis results or symptoms. Routine chest radiographs have a yield of 6% to 12.5% and should be obtained in patients with and without signs and symptoms localizing to the lung. Blood cultures can also be obtained but will most likely be of little benefit unless the patient has a high fever (102°F). In patients with costovertebral angle tenderness, CT scan, renal ultrasonography, or IVP may be indicated to rule out the presence of ureteral damage or obstruction from surgery, particularly in the absence of laboratory evidence of UTI. Patients who have persistent fevers without a clear localizing source should undergo CT scanning of the abdomen and pelvis to rule out the presence of an intraabdominal abscess. Finally, in patients who have had GI surgery, a CT scan with oral contrast, a barium enema, or an upper GI series with small bowel follow-through may be indicated late in the course of the first postoperative week if fever persists to rule out an anastomotic leak or fistula.
Urinary tract infections.
Historically, the urinary tract has been the most common site of infection in surgical patients. However, the incidence reported in the more recent gynecologic literature has been less than 4%. This decrease in UTIs is most likely the result of increased perioperative use of prophylactic antibiotics.
Symptoms of a UTI may include urinary frequency, urgency, and dysuria. In patients with pyelonephritis, other symptoms include flank pain, headache, malaise, nausea, and vomiting. A UTI is diagnosed on the basis of microbiology and has been defined as the growth of 10 5 organisms/mL urine cultured. Most infections are caused by coliform bacteria, with Escherichia coli being the most common pathogen. Other pathogens include Klebsiella , Proteus , and Enterobacter spp. Staphylococcus organisms are the causative bacteria in fewer than 10% of cases.
Despite the high incidence of UTIs in the postoperative period, few of these infections are serious. Most are confined to the lower urinary tract, and pyelonephritis is a rare complication. Catheterization of the urinary tract, either intermittently or continuously with the use of an indwelling catheter, has been implicated as a main cause of urinary tract contamination.
The treatment of UTI includes hydration and antibiotic therapy. Commonly prescribed and effective antibiotics include sulfonamide, cephalosporins, fluoroquinolones, and nitrofurantoin. The choice of antibiotic should be based on knowledge of the susceptibility of organisms cultured at a particular institution. In some institutions, for example, more than 40% of E. coli strains are resistant to ampicillin. For uncomplicated UTIs, an antibiotic that has good activity against E. coli should be given in the interim while awaiting the urine culture and sensitivity data.
Patients who have a history of recurrent UTIs, those with chronic indwelling catheters (Foley catheters or ureteral stents), and those who have urinary conduits should be treated with antibiotics that will be effective against the less common urinary pathogens such as Klebsiella and Pseudomonas spp . Chronic use of the fluoroquinolones for prophylaxis is not advised because these agents are notorious for inducing antibiotic-resistant strains of bacteria.
Pulmonary infections.
The respiratory tract is a relatively common site for complications after surgery for gynecologic cancer. Risk factors include extensive or prolonged atelectasis, preexistent chronic obstructive pulmonary disease, severe or debilitating illness, central neurologic disease causing an inability to clear oropharyngeal secretions effectively, and NG suction. In surgical patients, early ambulation and aggressive management of atelectasis are the most important preventive measures.
A significant proportion (40%–50%) of hospital-acquired pneumonias are caused by Gram-negative organisms. These organisms gain access to the respiratory tract from the oral pharynx. Gram-negative colonization of the oral pharynx has been shown to be increased in patients in acute care facilities and has been associated with the presence of NG tubes, preexisting respiratory disease, mechanical ventilation, tracheal intubation, and paralytic ileus (which is associated with microbial overgrowth in the stomach).
A thorough lung examination should be included in the assessment of all febrile surgical patients. In the absence of significant lung findings, chest radiography should nonetheless be obtained in patients at high risk for pulmonary complications. A sputum sample should also be obtained for Gram stain and culture. The treatment should include postural drainage, aggressive pulmonary toilet, and antibiotics. The antibiotic chosen should be effective against both Gram-positive and Gram-negative organisms, and in patients who are receiving assisted ventilation, the antibiotic spectrum should include drugs that are active against Pseudomonas organisms.
Wound infections.
The results of a prospective study of more than 62,000 wounds are revealing in regard to the epidemiology of wound infections. The wound infection rate varied markedly, depending on the extent of contamination of the surgical field. The wound infection rate for clean surgical cases (infection not present in the surgical field, no break in aseptic technique, no viscus entered) was lower than 2%, but the incidence of wound infections with dirty, infected cases was 40% or higher. Preoperative showers with hexachlorophene slightly lowered the infection rate for clean wounds, but preoperative shaving of the wound site with a razor (but not clippers) increased the infection rate. A 5-minute wound preparation immediately before surgery was as effective as preparation 10 minutes before surgery. The wound infection rate increased with the duration of the preoperative hospital stay and with the duration of the surgical procedure. (Prophylactic antibiotics should be readministered if the surgical procedure lasts longer than 4 hours.) In addition, incidental appendectomy increased the risk of wound infection in patients undergoing clean surgical procedures. The study concluded that the incidence of wound infections could be decreased by short preoperative hospital stays, hexachlorophene showers before surgery, clipping hair (not shaving) of the wound site immediately before incision, use of meticulous surgical technique, decreasing operative time as much as possible, bringing drains out through sites other than the wound, and dissemination of information to surgeons regarding their wound infection rates. A program instituting these conclusions led to a fall in the clean wound infection rate from 2.5% to 0.6% over an 8-year period. Although the wound infection rate in most gynecologic services has been lower than 5%, reflective of the “clean” nature of most gynecologic operations, it is higher in gynecologic oncology patients.
The symptoms of wound infection often occur late in the postoperative period, usually after the fourth postoperative day, and may include the presence of fever, erythema, tenderness, induration, and purulent wound drainage. Wound infections that occur on postoperative days 1 through 3 are generally caused by streptococcal and clostridial infections. The management of wound infections is mostly mechanical and involves opening the infected portion of the wound above the fascia, with cleansing and débridement of the wound edges as necessary. Wound care, consisting of débridement and dressing changes two to three times daily with mesh gauze, will promote growth of granulation tissue, with gradual filling in of the wound defect by secondary intention. The application of a Wound-Vac (negative pressure wound therapy) to larger wounds speeds recovery and minimizes dressing changes. In a gynecologic oncology population, the median number of days of wound vac therapy was 32 days (range, 3–88 days) with a 96% reduction in the median size of the wounds. Clean, granulating wounds can often be secondarily closed with good success, shortening the time required for complete wound healing.
The technique of delayed primary wound closure can be used in contaminated surgical cases to reduce the incidence of wound infection. Briefly, this technique involves leaving the wound open above the fascia at the time of the initial surgical procedure. Vertical interrupted mattress sutures though the skin and subcutaneous layers are placed 3 cm apart but are not tied. Wound care is instituted immediately after surgery and continued until the wound is noted to be granulating well. Sutures may then be tied, and the skin edges can be further approximated using sutures or staples. Using this technique of delayed primary wound closure, the overall wound infection rate has been shown to be decreased from 23% to 2.1% in high-risk patients.
Vaginal cuff infection after hysterectomy is characterized by erythema, induration, and tenderness at the vaginal cuff. Occasionally, a purulent discharge from the apex of the vagina may also be present. The cellulitis is often self-limited and does not require any treatment. Fever, leukocytosis, and pain localized to the pelvis may accompany severe cuff cellulitis and most often signifies extension of the cellulitis to adjacent pelvic tissues. In such cases, broad-spectrum antibiotic therapy should be instituted with coverage for Gram-negative, Gram-positive, and anaerobic organisms. If purulence at the vaginal cuff is excessive or if there is a fluctuant mass noted at the vaginal cuff, the vaginal cuff should be gently probed and opened with a blunt instrument. The cuff can then be left open for dependent drainage.
Intraabdominal and pelvic abscess.
The development of an abscess in the surgical field or elsewhere in the abdominal cavity is most likely to occur in contaminated cases in which the surgical site is not adequately drained or as a secondary complication of hematoma that becomes infected. The causative pathogens in patients who have intraabdominal abscess are usually polymicrobial in nature. The aerobes most commonly identified include E. coli and Klebsiella, Streptococcus, Proteus, and Enterobacter spp. Anaerobic isolates are also common, usually from the Bacteroides group. These pathogens are mainly from the vagina but also can be derived from the GI tract, particularly when the colon has been entered at the time of surgery.
An intraabdominal abscess is sometimes difficult to diagnose. The evolving clinical picture is often one of persistent febrile episodes with a rising white blood cell count. Findings on abdominal examination may be equivocal. If an abscess is located deep in the pelvis, it may be palpable by pelvic or rectal examination. For abscesses above the pelvis, diagnosis will depend on radiologic confirmation.
Ultrasonography can occasionally delineate fluid collections in the upper abdomen and the pelvis. However, bowel gas interference makes visualization of fluid collections or abscesses in the mid-abdomen difficult to distinguish. CT scanning with oral and IV contrast is much more sensitive and specific for diagnosing intraabdominal abscesses and is the radiologic procedure of choice.
Standard therapy for an intraabdominal abscess is drainage combined with appropriate parenteral administration of antibiotics. Abscesses located low in the pelvis, particularly in the area of the vaginal cuff, can often be reached through a vaginal approach. In many patients, the ability to drain an abscess by placement of a drain percutaneously under CT guidance has obviated the need for surgical exploration. With CT guidance, a pigtail catheter is placed into an abscess cavity via percutaneous, transperineal, transrectal, or transvaginal approaches. The catheter is left in place and irrigated daily until drainage decreases. Transperineal and transrectal drainage of deep pelvic abscesses has been successful in 90% to 93% of patients, obviating the need for surgical management. However, for patients in whom radiologic drainage is not successful, surgical exploration and evacuation are indicated. The gold standard of initial antibiotic therapy has been the combination of ampicillin, gentamycin, and clindamycin. Adequate treatment can also be achieved with currently available broad-spectrum single agents (including the broad-spectrum penicillin), second- and third-generation cephalosporins, levofloxacin and metronidazole, and the sulbactam/clavulanic acid–containing preparations.
Necrotizing fasciitis.
Necrotizing fasciitis is an uncommon infectious disorder; approximately 1000 cases occur annually in the United States. The disorder is characterized by a rapidly progressive bacterial infection involving the subcutaneous tissues and fascia while characteristically sparing underlying muscle. Systemic toxicity is a common feature of this disease, as manifested by the presence of dehydration, septic shock, disseminated intravascular coagulation, and multiorgan system failure.
The pathogenesis of necrotizing fasciitis involves a polymicrobial infection of the dermis and subcutaneous tissue. Historically, hemolytic streptococcus was believed to be the primary pathogen responsible for necrotizing fasciitis. However, it is now evident that numerous other organisms are present in addition to streptococcus, including other Gram-positive organisms, coliforms, and anaerobes. Bacterial enzymes such as hyaluronidase and lipase released in the subcutaneous space destroy the fascia and adipose tissue and induce a liquefactive necrosis. In addition, noninflammatory intravascular coagulation or thrombosis subsequently occurs. Intravascular coagulation results in ischemia and necrosis of the subcutaneous tissues and skin. Late in the course of the infection, destruction of the superficial nerves produces anesthesia in the involved skin. The release of bacteria and bacterial toxins into the systemic circulation results in septic shock, acid–base disturbances, and multiorgan impairment.
The diagnostic criteria for necrotizing fasciitis include extensive necrosis of the superficial fascia and subcutaneous tissue with peripheral undermining of the normal skin, a moderate to severe systemic toxic reaction, the absence of muscle involvement, the absence of clostridia in wound and blood culture, the absence of major vascular occlusion, intensive leukocytic infiltration, and necrosis of subcutaneous tissue.
Most patients with necrotizing fasciitis suffer pain, which in the early stages of the disease is often disproportionately greater than that expected from the degree of cellulitis present. Late in the course of the infection, the involved skin may actually be anesthetized secondary to necrosis of superficial nerves. Temperature abnormalities, both hyperthermia and hypothermia, are concomitant with the release of bacterial toxins and with bacterial sepsis, which is present in up to 40% of patients. The involved skin is initially tender, erythematous, and warm. Edema develops, and the erythema spreads diffusely, fading into normal skin, characteristically without distinct margins or induration. Subcutaneous microvascular thrombosis induces ischemia in the skin, which becomes cyanotic and blistered. As necrosis progresses, the skin becomes gangrenous and may slough spontaneously. Most patients will have leukocytosis and acid–base abnormalities. Finally, subcutaneous gas may develop, which can be identified by palpation (crepitus) and by radiograph. The finding of subcutaneous gas by radiograph is often indicative of clostridial infection, although it is not a specific finding and may be caused by other organisms. These organisms include Enterobacter spp. , Pseudomonas spp. , anaerobic streptococci, and Bacteroides spp., which, unlike clostridial infections, spare the muscles underlying the affected area. A tissue biopsy specimen for Gram stain and aerobic and anaerobic culture should be obtained from the necrotic center of the lesion to identify the causative organisms.
Predisposing risk factors for necrotizing fasciitis include diabetes mellitus, alcoholism, an immunocompromised state, hypertension, peripheral vascular disease, IV drug abuse, and obesity. Increased age, delay in diagnosis, inadequate debridement during initial surgery, extent of disease on initial presentation, and the presence of diabetes mellitus are all factors that have been associated with an increased likelihood of mortality from necrotizing fasciitis.
Early diagnosis and aggressive management of this lethal disease have led to improved survival. In an earlier series, the mortality rate was consistently higher than 30%, but in more recent series, the mortality rate has decreased to less than 10%.
Successful management of necrotizing fasciitis involves early recognition, immediate initiation of resuscitative measures (including correction of fluid, acid–base, electrolyte, and hematologic abnormalities), aggressive surgical debridement (and redebridement as necessary), and broad-spectrum antibiotic therapy. Many patients benefit from central venous monitoring and high caloric nutritional support.
During surgery, the incision should be made through the infected tissue down to the fascia. An ability to undermine the skin and subcutaneous tissues with digital palpation often confirms the diagnosis. Multiple incisions can be made sequentially toward the periphery of the affected tissue until well-vascularized, healthy, resistant tissue is reached at all margins. The remaining affected tissue must be excised. The wound can then be packed and sequentially debrided on a daily basis as necessary until healthy tissue is displayed at all margins.
Hyperbaric oxygen therapy historically was considered to benefit, particularly in patients for whom culture results are positive for anaerobic organisms. Retrospective studies demonstrated that the addition of hyperbaric oxygen therapy to surgical debridement and antimicrobial therapy appears to significantly decrease both wound morbidity and overall mortality in patients with necrotizing fasciitis. However, more contemporary, large-scale studies have failed to show a benefit of this treatment. Given the technical difficulty with its administration, hyperbaric oxygen treatment should only be considered in hemodynamically stable patients for whom the treatment will not delay surgical management.
After the initial resuscitative efforts and surgical debridement, the primary concern is the management of the open wound. Allograft and xenograft skin can be used to cover open wounds, thus decreasing heat and evaporative water loss. Interestingly, temporary biologic closure of open wounds also seems to decrease bacterial growth. Application of a wound vacuum-assisted closure (VAC) device will also promote wound healing. When spontaneous closure is not likely, the VAC device may allow for the development of a suitable granulation bed and prepare the tissue for graft placement, thereby increasing the probability of graft survival. Finally, skin flaps can be mobilized to help cover open wounds after the wound infections have resolved and granulation has begun.
Special Populations
Obesity
Incidence and definition.
Obesity (BMI ≥30) is an epidemic in the United States and is commonly encountered as a risk factor for complications associated with gynecologic oncology surgery. Centers for Disease Control and Prevention statistics from the year 2014 indicate that 34.9% of US adults (and 36.1% of women) older than age 20 years are obese. Current American Gastroenterology Association Guidelines use BMI to define classes of obesity (overweight, 25–29.9; class I, 30–34.9; class II, 35–39.9; and class III, >40.0). Of importance to the gynecologic oncologists are the further classes of morbid obesity (BMI >40) and super morbid obesity (BMI >50).
Postoperative complications and management.
Obese patients are at much higher risk for postoperative complications because of the more common occurrence of comorbidities, including diabetes, hypertension, coronary artery disease, sleep apnea, obesity hypoventilation syndrome (OHS), and osteoarthritis of the knees and hips. These underlying alterations in physiology result in increased surgical risks and complications, including respiratory failure, cardiac failure, DVT and pulmonary embolism, aspiration, wound infection and dehiscence, postoperative asphyxia, and misdiagnosed intraabdominal catastrophe.
Control of the airway is critical in the immediate postoperative period. Extubation may not be prudent or possible at the end of the case as a result of tracheal edema resulting from a difficult intubation. Alternatively, the patient may not have the physical capacity to adequately ventilate as a result of suppression of the respiratory drive from anesthetics and excess chest wall weight. Many obese patients have OHS, which increases their baseline hypercarbia and may also delay extubation. It is may be necessary to plan immediate postoperative admission to a surgical ICU with mechanical ventilation and serial arterial blood sampling to aid in the proper timing for extubation.
After extubation, ventilation of the obese patient during sleep may be aided by the use of noninvasive positive-pressure ventilation units, particularly if the patient has a history of sleep apnea and uses a continuous positive airway pressure (CPAP) machine at home. Respiratory therapists can be of assistance in patient instruction and management of CPAP machinery in addition to other respiratory toilet. Monitoring with continuous pulse oximetry assists in the detection of impending respiratory failure.
There is a higher risk of aspiration in obese patients due to increased gastric residual volumes, a higher rate of gastroesophageal reflux disease (GERD), and increased intraabdominal pressure from mass effect. Neutralization of the stomach contents with a proton pump inhibitor can minimize the chemical burn potential of aspirated stomach contents. GI motility agents such as metoclopramide may decrease residual volume by increasing intestinal transit. It is also prudent to raise the head of the bed to prevent aspiration.
Prophylaxis for postoperative venous thrombosis and pulmonary embolism (as detailed earlier) should be ordered for obese patients because they are at higher risk for these complications.
Venous access poses another problem. Extreme obesity obliterates anatomic landmarks and makes insertion of peripheral lines and central lines problematic. Adjunctive visualization technology such as Doppler ultrasonography or fluoroscopy should increase the accuracy and safety of line placement. Arterial line placement facilitates monitoring of pressures and blood gas parameters. Ideally, central venous lines and arterial lines should be placed intraoperatively by the anesthesia team to ensure adequate access in the postoperative period. The intraoperative placement of central lines should be verified for position postoperatively by chest radiography in the postanesthesia care unit.
Medication administration must consider the concepts of total body weight and ideal body weight. Certain medications are dosed on ideal body weight (corticosteroids, penicillin, cephalosporins, beta-blockers). Others are dosed on total body weight (heparin), and still others are based on a calculated “dosing weight” (aminoglycosides, fluoroquinolones, vancomycin). An inpatient pharmacist should be consulted for assistance with proper dosing and monitoring of pharmacotherapy.
Minimally invasive surgical techniques decrease the morbidity of surgery in obese patients. In particular, wound complications are greatly reduced, and patients are able to ambulate sooner and have a faster return to full levels of activity. This is particularly important in the endometrial cancer population, for whom obesity is such a strong driver of disease. Concerns regarding longer operating times and the feasibility of steep Trendelenburg positioning were initially raised in this population. A recent multi-institution retrospective cohort study of 1032 obese (BMI >30) patients who all underwent robotic endometrial cancer staging reported a pulmonary complication rate of just 3% ( n = 33) and overall complication rate of 14% ( n = 146), both significantly less than that of laparotomy. Several more large studies have consistently reported decreased complication rates with equal or improved comprehensive surgical staging rates for obese patients undergoing minimally invasive surgery. Minimally invasive surgery has been endorsed by the Society of Gynecologic Oncology as the preferred surgical approach for all, including obese, patients with endometrial cancer.
Elderly patients.
The life expectancy of American women continues to increase. In addition, as the baby boomer generation ages, we will see an increasingly larger population of older women who will develop gynecologic cancers. Elderly women are at higher risk of developing gynecologic cancer in that 57% of vulvar cancers, 55% of epithelial ovarian cancers, 41% of endometrial cancers, and 20% of cervical cancers occur in women older than 65 years of age. Although many of these cancers are managed with radical surgery, careful consideration must be given in selection of surgical procedures in women who may be at high risk for surgical complications. In addition to patient’s chronological age, preoperative evaluations that include measurements of frailty, performance status, quality of life, and geriatric assessments can aid greatly in appropriate patient selection for radical surgery. In fact, complications of radiation therapy and chemotherapy are more likely to occur in older patients, so selection of any therapy requires consideration of patient tolerance. In past years, radical surgery was avoided in elderly women. We now know that surgery can be safely and successfully accomplished, despite a patient’s age, if the patient is fully evaluated and determined to be a surgical candidate. There is no doubt that older patients present with more advanced disease and have poorer preoperative performance status and more comorbid conditions than younger patients. Retrospective review of elderly patients with gynecologic cancers demonstrates that 90% of women older than age 65 years can undergo radical surgery as definitive therapy for their gynecologic cancers. Compared with women younger than 65 years, the postoperative mortality rate was 1.5%, and the minor complications and length of stay were similar in the two groups. In women older than 65 years of age who underwent radical hysterectomy for early-stage cervical cancer, there was no perioperative mortality or ureteral fistula. Transfusion requirements and lymphedema were also similar. Febrile morbidity was less common in the older patients, although postoperative small bowel obstruction, bladder dysfunction, and pulmonary emboli were more commonly encountered in the older patients. In a population-based evaluation of 28,651 women undergoing cytoreductive surgery for ovarian cancer across the country, age was significantly associated with increased complication rate (<50 years, 17%; >70 years, ≥30%), including surgical site, medical, and infectious complications. With regard to minimally invasive surgery, in contrast, there is minimal increase in morbidity associated with age. Elderly cohorts of patients undergoing robotic surgery for endometrial cancer have been reported to have equivalent outcomes of blood transfusions, surgical complications, and length of hospital stay while preserving surgical comprehensive staging.
Perioperative management is the key to success when managing elderly women. Cardiac and pulmonary complications are the two most common serious problems encountered postoperatively. Careful preoperative assessment of cardiac status should include assessment for underlying coronary artery disease, valvular heart disease, and chronic congestive heart failure. Several risk-assessment algorithms may be applied to estimate cardiac risk of major noncardiac surgery. Because more than 40% of elderly women have hypertension, optimal blood pressure control should be achieved preoperatively; intraoperative hypotension is one of the most common causes of myocardial ischemia and infarction. Pulmonary complications occur in nearly 40% of elderly women after major abdominal surgery. Because physiologic changes of aging diminish vital capacity, lung compliance, reduced expiratory flow rates, and increased residual volume, elderly patients are more likely to have pulmonary complications after general anesthesia. These women may be at even higher risk if they have chronic obstructive pulmonary disease or asthma. Preoperative assessment may include assessment of pulmonary function by performing spirometry and obtaining an arterial blood gas measurement. Patients with underlying pulmonary disease should have their medical regimen maximized preoperatively, including the use of bronchodilators and corticosteroids. Conduction anesthesia should be considered in consultation with an anesthesiologist to avoid the pulmonary complications more often encountered with general anesthesia.
Avoiding perioperative hypothermia and hypoxemia are extremely important to avoid additional cardiac oxygen consumption. Invasive monitoring and planned ICU admission should be considered in any circumstance when there is an increased risk of cardiac or pulmonary complications. Care must also be taken when ordering pharmacologic agents because elderly patients may have altered GI absorption and decreased renal or hepatic clearance of specific drugs. Consultation with a clinical pharmacist is advised to establish the correct dose of drug for patients with altered renal or hepatic function.
Radiation complications in elderly patients are also increased. Thin, hypertensive patients appear to be at greater risk for radiation therapy complications to both the GI and genitourinary tracts. It appears that elderly women tolerate initial therapy poorly and often require delays in treatment or discontinuation of treatment because of acute toxicity such as diarrhea, dehydration, or neutropenia. This observation, then, requires carefully planned treatment decisions as to whether the patient would be best treated with surgery or radiation therapy. Neither is without risk, and it cannot be assumed that radiation therapy is necessarily less toxic.
Radiation Therapy
Radiation therapy serves as a primary treatment modality for cervical and vaginal cancers (and occasionally advanced vulvar cancers) and as an adjuvant therapy for patients with high-risk endometrial cancers. Furthermore, individualized radiation therapy may be used in nearly all gynecologic cancers to achieve palliation under specific circumstances. Morbidity resulting from properly conducted radiation therapy in patients with carcinoma of the cervix and vagina is usually minimal. However, there are unfortunate misconceptions about the magnitude of radiation morbidity because of issues regarding failure to distinguish between adverse effects from poor techniques, failure to recognize that poorer outcomes of patients with inoperable extensive tumors, and lack of recognition that morbidity attributed to irradiation may actually result from uncontrolled tumor (ie, rectovaginal and vesicovaginal fistulas). As in the case of surgery, the treatment-related morbidity can be minimized by good application, but it cannot be eliminated.
Because the small bowel, bladder, and rectum are adjacent to the female genital tract, most of the side effects and complications of radiation involve these adjacent organs. Radiation complications are related to the dose, field size, and type of radiation equipment used. The larger the field, the greater the risk of problems if the dose remains constant. Usually, as the fields enlarge, the dose must be decreased. Conversely, as the fields become smaller, a larger dose can be tolerated. The use of brachytherapy also increases the risk of local complications. Finally, the use of combined chemoradiation therapy seems to increase slightly some complications during therapy (eg, neutropenia) but does not seem to lead to serious long-term sequelae.
The pathogenesis of radiation-induced injury may be divided into acute and delayed complications. Complications during therapy are caused by ionizing radiation injury to cells that are mitotically active such as the GI epithelium. Damage of the mucosal cells results in mucosal thinning and denudation followed by malabsorption and fluid and electrolyte loss (resulting from diarrhea). The GI mucosal stem cells generally recover totally, and the acute symptoms resolve. Late complications involve a different mechanism of tissue injury based on vascular endothelial damage. Radiation results in an endarteritis and the gradual occlusion of small vessels. Subsequent tissue hypoxia leads to fibrosis of the affected tissue. These changes are progressive and may be aggravated further by other vascular compromise such as diabetes, hypertension, and aging. In severe cases, ulceration, stricture, perforation, and fistula formation may occur.
Gastrointestinal Complications
Acute complications.
Nearly all patients receiving external radiation to the pelvis will develop radiation proctitis or enteritis with associated diarrhea. This problem commonly begins after 2 weeks of radiation therapy and is usually easily managed by dietary modification and antidiarrheal medications (diphenoxylate; Lomotil). As a rule, the diarrhea resolves within 7 to 10 days of completing radiation. Some of the transitory symptoms are tenesmus and the passage of mucus and even blood per the rectum. Diarrhea and abdominal cramping characterize small intestinal irritation (radiation enteritis). This problem is more common and more severe when a portion of the small intestine is fixed in the pelvis as a result of adhesions or other pathologic conditions. Anorexia may also occur during radiation therapy. If nausea and vomiting occur, the patient should be evaluated for dehydration. This is more common when concurrent chemotherapy is being given along with the radiation. IV hydration and correction of electrolytes is occasionally required. If dehydration is severe, radiation should be interrupted until these acute side effects are corrected.
The patient should have a complete blood count weekly during radiation therapy. If the hemoglobin decreases below 10 g/dL, PRBC transfusion is advised to achieve improved tumor kill. Occasionally, radiation of the pelvic bone marrow will result in neutropenia or thrombocytopenia. In severe cases, radiation will need to be temporarily interrupted. In nearly all cases, the acute side effects of radiation therapy will resolve after the radiation course is completed.
Chronic conditions.
Radiation injury to the small intestine may manifest itself at any time after therapy and may vary from chronic diarrhea to small bowel obstruction or fistula. A typical patient with small bowel injury will initially present with postprandial abdominal cramps and pain, anorexia, and diarrhea. About half of small bowel injuries occur within 1 year after radiation, and three-fourths occur within 2 years. These symptoms are more common in patients who have had a prior laparotomy. Initial conservative management focuses on dietary modification (avoiding green leafy vegetables, milk products, and fried foods). Antidiarrheal drugs and oral cholestyramine (which binds bile salts) are often useful in managing symptoms.
As small bowel injury progresses, fibrosis of the injured bowel wall develops, leading to stricture and partial or complete obstruction. Patients usually present with worsening abdominal pain, cramps, diarrhea, and vomiting. Sometimes the intermittent obstruction goes unrecognized until a significant weight loss is recorded. If medical management of a partial small bowel obstruction fails, surgical intervention is the course of last resort. The decision to proceed with surgical intervention in cases of intermittent obstruction is a difficult one and should be undertaken with proper preoperative preparation. Often, patients have developed a significant degree of malnutrition and should have their nutritional status corrected with preoperative TPN. Mechanical bowel preparation is mandatory, although sometimes it must be limited to enemas because the patient’s small bowel obstruction will not allow the ingestion of oral cathartics. Dilated loops of bowel should be decompressed preoperatively with NG suction. Surgical correction depends on the situation encountered at the time of surgical exploration. The obstruction is usually found to be bowel adherent in the pelvis that has been radiated. The bowel usually is thickened, edematous, and fibrotic. The two primary surgical options are resection of the obstructed loops with either reanastomosis or small bowel bypass. In either event, it is preferable to make anastomoses to intestinal segments that have not been radiated in order to have maximum opportunity for healing. Care should be taken to have adequate perfusion and no tension on the anastomosis. Perforation or fistula, if present, must be isolated and diverted; more extensive surgery (resection and anastomosis) must be done only if it is technically feasible ( Fig. 16.10 ).