Author
Overall recurrence rate
Local
Average time (month)
Regional
Average time (month)
5-year survival (%)
Vukadinovic et al. [7]
17.5
10.8
Ns
4.5
Ns
97.8
Grover et al. [8]
27.5
22.2
43
5.5
14
93
McGregor et al. [9]
20.5
2.5
Ns
18
12.4
Babington et al. [18]
31
20
12
13
12
91
Rowe et al. [10]
14.8
7.6
Ns
7.2
Ns
Cerezo et al. [11]
8.5
3.4
Ns
5.1
Ns
Casal et al. [3]
Ns
12.8
35.1
Ns
Ns
91.7
Zitsch et al. [4]
15.1
11.4
Ns
3.7
Ns
89
Saywell and Weeden [12]
35
8
18
27
12
de Visscher et al. [13]
17
4.6
Ns
12.4
29
Zitsch et al. [2]
16
12
Ns
4
Ns
McCombe et al. [14]
7.6
3.3
Ns
4.3
Ns
98
Veness et al. [15]
33
11.8
2.4
19.4
7.3
85
de Visscher et al. [5]
10.3
4.9
38
5.4
26
86
Papadopoulos et al. [16]
Ns
11.3
Ns
Ns
Ns
89
Gooris et al. [6]
Ns
6
Ns
Ns
Ns
Ns
Many of these studies retrospectively review a large number of cases performed by multiple surgeons over long periods of time. In most studies it was not stated if the National Comprehensive Cancer Network (NCCN) guidelines were used. Without a particular set of guidelines, treatment can vary from study to study in regard to surgical oncologic margins and indications for neck dissections and when adjunctive therapy should be used. This makes it very difficult to analyze these studies side by side, which is the inherent nature of retrospective studies. Therefore, the above-listed recurrence rates can be very useful but should be taken as a broad reference of probabilities. Future studies and treatment should follow the NCCN guidelines to make it easier to compare and quantify results.
The chance of recurrence varies due to a large number of prognostic factors. Tumor size, site, cervical nodal stage, histological grade, depth of invasion, margin status, and type of treatment all affect the overall outcome and the probabilities of recurrence.
Tumor site can be compartmentalized into lower lip, upper lip, commissure, or multisite involvement. Although cancer is more common in the lower lip, upper lip and commissure cancers are linked to a worse prognosis. There is a higher recurrence rate and a lower survival rate of 90 and 77 %, respectively [4]. NCCN guidelines do not indicate alterations in treatment based on location of lip cancers, but the more aggressive nature of upper lip or commissure involvement should not be overlooked. Possible future revisions of these guidelines may someday hold variations in treatment based on site.
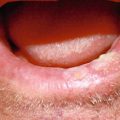
In addition to site, tumors of the lip can vary in size from a few millimeters to encompassing more than the entire lip (Fig. 11.1). This obviously affects treatment and reconstruction, but it is also an indicator of recurrence. Multiple studies conclude that an increase in tumor size from a T1 lesion to T2 or larger increases recurrence rate by over sevenfold [13, 17]. Conversely some studies showed a much smaller difference in recurrence rate [15] or no difference at all between tumor size and recurrence rate [4]. Although not linking size to recurrence, these studies did indicate increasing tumor size was predictive of poorer overall survival rate [6, 18] and of cervical nodal metastasis [2]. Cervical nodal metastasis was seen in 7 % of T1 tumors and 16 % of T2–T4 tumors [2]. Also the 10-year probability of cervical nodal metastasis was 4 % in T1 tumors compared to 20 % for T2 or T3 tumors [11].
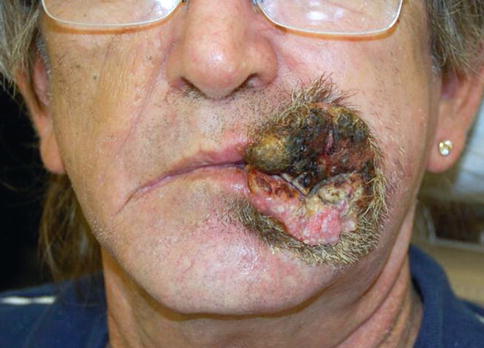

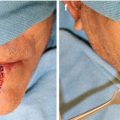
Fig. 11.1
Large squamous cell carcinoma encompassing upper lip, commissure, and lower lip
Tumor thickness also shows a very strong correlation with nodal metastasis. There is a statistically significant difference between tumor thicknesses of less than or greater than 5–6 mm for predicting lymph node metastasis [19, 20]. It was shown that there was almost a 20-fold increase in lymph node metastases in patients with 6 mm or greater depth of invasion compared to tumors with invasion less than 6 mm [20].
As stated previously, lip cancer is usually treated surgically. This makes positive margins one of the first complications encountered. It is usually discovered intraoperatively via frozen sections or very early in the postoperative period. If known intraoperatively, then further resection is indicated until negative margins are obtained. Even with negative frozen margins, the final pathology may show positive or close margins (Fig. 11.2). When this occurs, either adjunctive radiotherapy or re-resection is indicated according to NCCN guidelines.
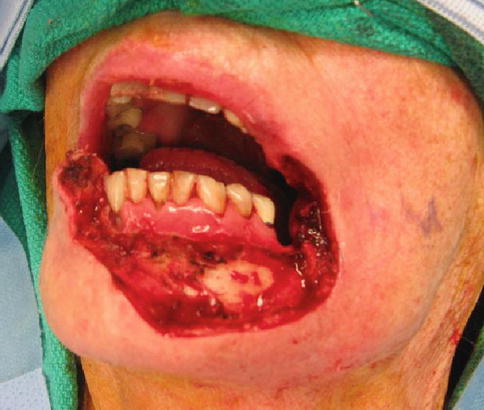

Fig. 11.2
Large resection with negative frozen margins, but positive margins on permanent pathology
The rate of incomplete excision of squamous cell carcinoma in the human body is 17.58 % [21]. There is a similar rate of 17.6 % [21] for the head and neck region. The rate of incomplete excision of lip carcinoma appears to be lower (3.1–27 %) [3–6, 18] with an increased risk in upper lip carcinomas [21]. This incidence of incomplete excision increases linearly with an increase in tumor size [21]. Patients with positive margins have an increased risk of local recurrence (38 % [5]) and a decreased survival rate when surgical margins were less than 2 mm [18]. These high recurrence rates make it easy to reiterate the necessity for close follow-up in the postoperative period.
Later in the postoperative course, there may be local recurrence, second primaries, and regional or distant metastasis. Any one of these outcomes can occur from poor surgical treatment planning and resection; unfortunately they also can occur when an adequate surgical resection has been completed. Local recurrence and regional or distant metastases all ultimately lead to a decreased 5-year survival rate [2, 22]. Proper initial staging and resection combined with close follow-up is the key to preventing and properly managing these negative consequences and should have priority over any plans of restoring function or esthetics.
Once a local recurrence is diagnosed, it must be treated, most often surgically with or without adjunctive chemotherapy and radiation therapy but sometimes with adjunctive therapy alone. When the impact of local recurrence on survival in patients with oral squamous cell carcinoma was reviewed, the 5-year survival rate significantly decreased to 31.5 % [22]. There was a considerable difference in the rate between patients treated surgically after recurrence (34.2 %) and those treated with adjunctive therapy alone (20.7 %). This was also affected by the time of recurrence after initial treatment; those who recurred in less than 18 months showed a 27.5 % 5-year survival versus 38.2 % for recurrences occurring 18 months or greater. The 5-year survival rate after recurrence of an isolated lip carcinoma was significantly higher (43.7 %) than for oral carcinoma overall (31.5 %) [22]. These low survival rates point to the destructive nature of this disease and the indication for aggressive surgical and adjunctive treatment.
In addition to local recurrence, second primaries can occur on the lip or in other areas of the body after head and neck cancer treatment. Second primaries occur after oral pharyngeal carcinomas in 20 % of patients [23]. This seems like a high percentage, but the common risk factors of HPV, tobacco use, alcohol use, genetics, and sun exposure may lead to these outcomes. This is mildly reduced to 17 % [24] when the primary cancer originates from the lip. The reduction may be due the fact that alcohol abuse and tobacco use do not play a major role in lip cancer development. There is also a 3.3 % [5] rate of second primaries on the lip after initial treatment for lip cancer. These second primaries have been shown to occur in multiple areas, lip, oral cavity, lung, bladder, stomach, breast, and prostate, but could be seen in other locations as well. Second primaries need to be differentiated from metastasis of primary disease by biopsy prior to further treatment. Once confirmed, second primaries are treated with same NCCN standard guidelines as the original primary. It is recommended to have lifelong follow-up of these patients with periodic panendoscopy especially in patients who exhibit high-risk behavior.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree