Multimodality treatment of rectal cancer, with the combination of radiation therapy, chemotherapy, and surgery has become the preferred approach to locally advanced rectal cancer. The use of neoadjuvant chemoradiation therapy (CRT) has resulted in reduced toxicity rates, significant tumor downsizing and downstaging, better chance of sphincter preservation, and improved functional results. A proportion of patients treated with neoadjuvant CRT may ultimately develop complete clinical response. Management of these patients with complete clinical response remains controversial and approaches including radical resection, transanal local excision, and observation alone without immediate surgery have been proposed. The use of strict selection criteria of patients after neoadjuvant CRT has resulted in excellent long-term results with no oncological compromise after observation alone in patients with complete clinical response. Recurrences are detectable by clinical assessment and frequently amenable to salvage procedures.
Multimodality treatment of rectal cancer, with the combination of radiation therapy, chemotherapy, and surgery has become the preferred approach to locally advanced rectal cancer. The considerably high local recurrence rates observed after radical surgery alone has led to the use and recommendation for additional therapy either before or after surgery for T3/T4 or N+ tumors. In this setting, to avoid overtreatment of patients with early-stage disease, preoperative treatment with radiation therapy with or without concomitant chemotherapy requires optimal radiological staging because there is no pathologic confirmation of exact TNM parameters. However, there is a theoretic benefit of exposing unscarred tissue with optimal oxygen delivery to both radiation and chemotherapy as opposed to postoperative treatment. The results from randomized controlled trials suggest that the neoadjuvant approach seems to be superior for local disease control, even in the setting of appropriate surgical technique (total mesorectal excision). The use of neoadjuvant chemoradiation therapy (CRT) has resulted in additional benefits such as reduced toxicity rates, significant tumor downsizing and downstaging, better chance of sphincter preservation, and improved functional results (compared with postoperative CRT). In a multicenter study of patients undergoing neoadjuvant CRT for clinically stage II disease (staged by either endorectal ultrasound or magnetic resonance imaging), more than 20% of the patients staged as N0 were found to harbor lymph node metastases in their tumors on pathologic examination. Considering that these patients underwent neoadjuvant CRT, even greater rates of nodal disease underestimation might be expected. The investigators concluded that radiological inaccuracy (particularly for nodal disease) may justify, and possibly warrant, overtreatment of patients with rectal cancer by the use of neoadjuvant CRT. There is a subset of patients with early-stage disease (particularly T2N0) who may also benefit from neoadjuvant CRT despite there having been no demonstration of improved local disease control in randomized trials. Patients with distal T2N0 rectal cancers are at higher risk for developing local disease recurrence compared with the mid- and upper rectal locations. In addition to the potential benefits in terms of local disease control in this high-risk group of patients, neoadjuvant CRT could also improve the chance for sphincter preservation in these patients, allowing for ultralow or even intersphincteric resections.
Rationale for the investigation of a nonoperative approach
Radical surgery with total mesorectal excision remains the mainstay of treatment of distal rectal cancer and is considered by many to be necessary regardless of tumor response to neoadjuvant CRT. However, it has been associated with high rates of immediate morbidity and mortality. For immediate morbidity, an anastomotic leak is one of the most important complications and may occur in up to 12% of cases. Overall, perioperative mortality may reach 2% to 3% in patients managed by radical surgery. Perioperative mortality is significantly higher, reaching up to 13% of patients with anastomotic leaks, when temporary diversion is not performed. The requirement for a temporary stoma may add additional morbidity related to stoma closure and should be considered in the cumulative morbidity of rectal cancer management. Preoperative radiation may lead to a significant increase in the risk of leaks; however, prospective randomized trials have failed to demonstrate the differences described in previous retrospective analysis.
Tumor regression after neoadjuvant CRT may be observed not only in the primary tumor (within the rectal wall) but also in perirectal metastatic lymph nodes. This finding has been supported by the observation of a shift toward earlier disease staging in patients treated preoperatively with CRT, for whom the rates of stage II or III disease are markedly decreased compared with patients managed by surgery and postoperative CRT.
Tumor regression after neoadjuvant CRT may be complete, leading to an absence of residual neoplasia in the resected specimen, known as complete pathologic response or ypT0N0M0 (ypCR).
Therefore, the rationale for a nonoperative approach to patients with rectal cancer is to avoid a significantly morbid procedure in patients with complete tumor regression after neoadjuvant CRT.
Factors influencing tumor regression
In a review of phase II and phase III studies including variable regimens of neoadjuvant CRT, several factors were found to be associated with higher rates of ypCR after radical surgery. The use of fluorouracil (5-FU) by continuous venous infusion, the delivery of a radiation therapy dose higher than 45 Gy, and the use of an additional drug combine with 5-FU have all been associated with increased rates of ypCR.
Another factor that has frequently been associated with complete tumor regression is the interval between CRT completion and surgery. Radical surgery has traditionally been recommended to be performed 6 weeks after CRT completion. In the authors’ experience with neoadjuvant CRT, initial assessment of tumor response should be performed no less than 8 weeks after CRT completion. No strong evidence exists to guide optimal timing for response assessment for rectal cancer; however, retrospective data indicate that longer periods after CRT completion may be associated with higher rates of tumor downstaging. Several retrospective studies have shown that patients managed by radical surgery 7 to 8 weeks after CRT completion had increased rates of ypCR. In one of the studies, patients managed by surgery 7 weeks after CRT had improved outcomes. In a recent retrospective review of patients managed by neoadjuvant CRT in a single institution, there was a steep increase in ypCR rates when surgery was performed 7 weeks after CRT completion. These ypCR rates stabilized after 12 weeks from CRT completion.
An argument for performing surgery shortly (<8 weeks) after CRT completion is the risk of leaving the tumor in situ for prolonged periods of time, with potential metastatic dissemination of tumor cells during this period. Tumor cell death seems to be related to a process induced by ionizing radiation. It is thought that after exposure to a dose of 44 Gy, the metastatic potential of these tumors is significantly compromised because of the potential decrease in the overall number of surviving cells in the tumor. One recent study analyzed the interval between CRT and surgery and found that a prolonged interval (>8 weeks) between CRT and surgery may not have any associated oncologic compromise. In addition, these patients were associated with less postoperative morbidity, further supporting the safety of assessing tumor response at prolonged intervals.
Factors influencing tumor regression
In a review of phase II and phase III studies including variable regimens of neoadjuvant CRT, several factors were found to be associated with higher rates of ypCR after radical surgery. The use of fluorouracil (5-FU) by continuous venous infusion, the delivery of a radiation therapy dose higher than 45 Gy, and the use of an additional drug combine with 5-FU have all been associated with increased rates of ypCR.
Another factor that has frequently been associated with complete tumor regression is the interval between CRT completion and surgery. Radical surgery has traditionally been recommended to be performed 6 weeks after CRT completion. In the authors’ experience with neoadjuvant CRT, initial assessment of tumor response should be performed no less than 8 weeks after CRT completion. No strong evidence exists to guide optimal timing for response assessment for rectal cancer; however, retrospective data indicate that longer periods after CRT completion may be associated with higher rates of tumor downstaging. Several retrospective studies have shown that patients managed by radical surgery 7 to 8 weeks after CRT completion had increased rates of ypCR. In one of the studies, patients managed by surgery 7 weeks after CRT had improved outcomes. In a recent retrospective review of patients managed by neoadjuvant CRT in a single institution, there was a steep increase in ypCR rates when surgery was performed 7 weeks after CRT completion. These ypCR rates stabilized after 12 weeks from CRT completion.
An argument for performing surgery shortly (<8 weeks) after CRT completion is the risk of leaving the tumor in situ for prolonged periods of time, with potential metastatic dissemination of tumor cells during this period. Tumor cell death seems to be related to a process induced by ionizing radiation. It is thought that after exposure to a dose of 44 Gy, the metastatic potential of these tumors is significantly compromised because of the potential decrease in the overall number of surviving cells in the tumor. One recent study analyzed the interval between CRT and surgery and found that a prolonged interval (>8 weeks) between CRT and surgery may not have any associated oncologic compromise. In addition, these patients were associated with less postoperative morbidity, further supporting the safety of assessing tumor response at prolonged intervals.
Assessment of tumor response
Assessment of tumor response may be challenging for even the most experienced colorectal surgeon. Even though clinical symptoms subside in patients with complete clinical response, the specificity of symptoms is low because a significant proportion of patients experience some degree of tumor regression and symptom relief. However, residual disease may still be clinically obvious. Clinical assessment using digital rectal examination and rigid proctoscopy is the mainstay of response assessment. Until now, we have considered clinical assessment to be the major determinant for individualization of treatment strategies such as radical surgery, transanal local excision (particularly with the use of transanal endoscopic microsurgery), or even a nonoperative approach.
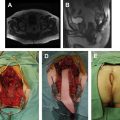
Complete clinical response is the absence of residual disease after neoadjuvant CRT. If there is any visible or palpable irregularity or nodule, even after near-complete tumor regression, full-thickness transanal local excision or transanal endoscopic microsurgery should be attempted as a diagnostic procedure. Besides proper surgical technique, full pathologic examination is of paramount importance, because diagnosis of microscopic residual disease may be a challenge for the pathologist. Such procedures should be performed by an experienced pathologist belonging to the multidisciplinary team involved in rectal cancer management. With the incorporation of the transanal endoscopic microsurgery technique, improved lateral and radial margin visualization during local excision may provide excellent specimens which will allow the pathologist to precisely determine margins, tumor regression grade, and other standard pathologic features ( Fig. 1 ). Forceps biopsies should not be routinely attempted in these patients, particularly in deciding between complete and incomplete response to neoadjuvant CRT, because the tissue specimens from this procedure are usually small and superficial, leading to an increased risk of false-negative results.
In our experience, a subtle whitening of the mucosa and the presence of telangiectasia may be detectable by proctoscopy in some patients with complete clinical regression, and these patients should be considered for an initial nonoperative approach ( Fig. 2 ).
Because clinical assessments by rigid proctoscopy and digital rectal examination are two of the most important tools in assessing tumor response to CRT, tumors located higher in the rectum may provide insufficient information for the alternative of a nonoperative approach. In addition, tumors that are not accessible to the surgeon’s finger may allow safe anterior resections with less risk for sphincter resection and local recurrence.
Studies performed on the accuracy of clinical assessment of patients with rectal cancer after neoadjuvant CRT showed disappointing sensitivity and specificity. However, these studies used 6-week intervals between CRT completion and response assessment, and therefore could have detected residual disease in patients with ongoing tumor regression. In addition, the inclusion of different examiners could have biased results, depending on an individual’s experience and perception of complete clinical responses.
Carcinoembryonic antigen (CEA) has been extensively used in colorectal cancer management for different purposes. One study of patients undergoing radical surgery alone for rectal cancer found that a decrease in CEA 7 days after rectal resection was associated with improved outcomes, suggesting that initial CEA levels were probably exclusively determined by the primary tumor instead of microscopic undetected metastatic disease. In addition to clinical and radiological assessment of tumor response, determination of CEA levels before and after CRT may also be useful. In a study of more than 500 patients with rectal cancer managed by neoadjuvant CRT, low CEA before treatment was a predictor of ypCR after radical surgery in univariate analysis. Similar findings have been reported in a retrospective analysis of patients undergoing variable neoadjuvant CRT regimens for very low (<2.5 ng/dL) pretreatment CEA levels. In our experience, the decrease or difference between pre-CRT and post-CRT CEA levels were not good predictors of complete tumor regression. Instead, patients with low post-CRT CEA levels, irrespective of pre-CRT CEA levels, had higher rates of complete clinical response and improved outcomes after neoadjuvant therapy.
Radiological assessment of tumor response has been the ultimate challenge in rectal cancer management. Staging of primary tumor depth of penetration and distance from the circumferential margin seem to be adequately provided by endorectal ultrasound and magnetic resonance imaging (metanalyses Santoro). However, in neoadjuvant CRT, distinguishing between residual cancer and transmural fibrosis may be significantly compromised by both ultrasonography and magnetic resonance imaging because these tools rely heavily on morphologic features. However, in neoadjuvant CRT, distinguishing between residual cancer and transmural fibrosis may be significantly compromised by both ultrasonography and magnetic resonance imaging because these tools rely heavily on morphologic features. Improvements in resolution with these pelvic imaging modalities may provide increased reliability of results in the near future.
The incorporation of positron emission tomography (PET) imaging into the staging work-up provided significant additional information by overlaying metabolic activity data with standard morphology. In addition, PET imaging may also provide an objective estimate of the metabolic activity of a specific area as represented by the standard uptake value measured at various phases of the study. One study of 25 patients with rectal cancer compared the results of baseline PET-computed tomography (CT) before CRT with a second PET-CT performed 6 weeks after CRT completion. All patients included in the study experienced a decrease in maximum standard uptake values (SUV max ) between baseline and 6-week PET-CT scans. Also, the final SUV max obtained at 6 weeks was significantly associated with primary tumor downstaging; patients with tumor downstaging exhibited significantly lower SUV max (1.9 vs 3.3; P = .03). Even though SUV max obtained at 6 weeks from CRT was associated with tumor downstaging, SUV max did not correlate with final outcome. In another study of 15 patients undergoing baseline PET (before CRT) followed by a second PET 6 weeks after CRT completion, the visual response score was shown to provide superior prediction of tumor downstaging in addition of the extent of pathologic response to CRT. This same group of patients was prospectively followed and outcome analysis showed that patients who developed recurrent disease had significantly lower percentual decrease between baseline and 6-week PET SUV max values. A cutoff of a 62.5% decrease/difference between baseline and 6-week PET SUV max values was a significant predictor of disease-free survival. The same investigators also studied the possibility of tumor response prediction by early sequential PET imaging. In a study of 25 patients undergoing baseline PET, followed by early (10 days after initiation of CRT) PET, the visual response score was able to predict patients who would develop ypCR after CRT. The visual response score could be useful in tailoring treatment of these patients.
These studies each included only a small number of patients, and none of them considered how increased interval periods between CRT and tumor response assessment may influence results. A more recent study examined 30 patients with rectal cancer less than 5 mm from the circumferential margin, as determined by magnetic resonance imaging. All patients underwent CRT, including 65.6 Gy of radiation, and baseline PET-CT and PET-CT performed 8 weeks after CRT were compared. This study found a poor correlation between PET-CT results and final pathologic findings. The sensitivity and specificity for ypCR were 75% and 40%, respectively. Twelve out of 30 patients had a false-negative PET-CT result.
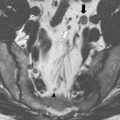
In an earlier study, we performed PET-CT studies in patients with complete clinical response and who were being managed nonoperatively after a variable interval of follow-up. In all 22 patients studied, PET-CT showed no signs of residual disease in the primary site, consistent with the findings of clinical, endoscopic, and radiological assessment. Eight patients with known residual disease after CRT completion also underwent PET-CT and served as a positive control group and all 8 patients were positive by PET-CT for residual disease within the rectal wall. This study suggests that PET-CT may be useful in late assessment of tumor response after nonoperative management of patients with complete clinical response after CRT. It also provides evidence of adequate long-term local and systemic control in patients with complete clinical response. There is an ongoing study at our institution, dedicated to the study of PET-CT in assessing tumor response after neoadjuvant CRT. In this study, all patients undergo a baseline, 6-week post-CRT, and a 12-week post-CRT PET-CT. In addition, patients with sustained complete clinical response and who are not operated on will undergo further PET imaging annually as part of follow-up. The results of this study are not available yet but should provide information on the role of PET-CT imaging in assessing tumor response, and on the benefits of a prolonged (12 weeks vs 6 weeks) interval of assessment in tumor metabolism and downstaging ( Figs. 3 and 4 ).