• Complementary and alternative medicine encompasses various approaches to all aspects of medical assessment and management that are not commonly or extensively applied or recommended by conventional western medical practitioners. • Complementary and alternative medicine approaches are frequently used by patients, but patients often do not discuss their use of these approaches with their health care team. • Some products purported to be dietary supplements may be unsafe for patients with cancer because of adverse effects of the natural components, adverse interactions with medications, contamination with toxic compounds, or adulteration with drugs. • St. John’s wort and potentially various other herbal products can significantly alter the pharmacokinetics of certain chemotherapy drugs, such as irinotecan. • High-dose alpha-tocopherol (i.e., 400 IU per day) should not be given to patients with head and neck cancer who are receiving radiation therapy with curative intent. • Acupuncture can be useful for managing cancer pain and postsurgical pain. • Exercise can be an effective intervention for preventing and managing cancer-related fatigue. • Yoga may be effective in preventing or managing fatigue and improving sleep and quality of life in patients with cancer. • Acupuncture, ginger, hypnosis, relaxation therapy, and imagery can provide additional relief from chemotherapy-induced nausea and vomiting in patients receiving standard antiemetic regimens. • High-dose oral glutamine and intravenous glutathione may decrease the frequency of neuropathy from drugs containing paclitaxel and platinum. • Preliminary evidence indicates that acupuncture may effectively decrease hot flash symptomatology in women undergoing treatment for breast cancer and in men receiving androgen-deprivation therapy for prostate cancer. • Aloe vera or honey taken orally and intravenously administered glutamine may decrease the incidence or severity of chemotherapy-induced oral mucositis. • Several interventions have been found useful for stress reduction and to increase the quality of life among patients with cancer, including music, meditation, relaxation therapy, and imagery therapy. Complementary and alternative medicine (CAM) is defined by the National Center for Complementary and Alternative Medicine (NCCAM), a component of the U.S. National Institutes of Health (NIH), as “a group of diverse medical and health care systems, practices, and products that are not generally considered part of conventional medicine,” with “conventional medicine” defined as “…medicine as practiced by holders of M.D. (medical doctor) and D.O. (doctor of osteopathic medicine) degrees and by allied health professionals, such as physical therapists, psychologists, and registered nurses.”1 The World Health Organization defines CAM as “a broad set of health care practices that are not part of that country’s own tradition and are not integrated into the dominant health care system.”2 It is generally accepted that complementary therapies are those that are used along with conventional medical care for a given condition and alternative therapies are those that are used independent of conventional care. Box 33-1 provides a categorization of CAM intervention and therapeutic systems using a modification of schema developed by NCCAM. A recent, large, national, population-based survey showed that 43.3% of U.S. patients with cancer had used at least one CAM intervention in the preceding 12 months.3 Evidence suggests that the use of such approaches, even those with the greatest theoretical potential for interaction with conventional therapy, is often not disclosed to the treating oncologist.4,5 Integrative medicine is defined by the Consortium of Academic Health Centers for Integrative Medicine as “an approach to the practice of medicine that makes use of the best-available evidence taking into account the whole person (body, mind, and spirit), including all aspects of lifestyle. It emphasizes the therapeutic relationship and makes use of both conventional and complementary/alternative approaches.”6 Patients with cancer often have unmet needs for dietary advice.7,8 Various small studies have shown that nutritional counseling and education improves outcomes (i.e., quality of life and/or survival) for patients with head and neck,9 colorectal,10 and lung cancer.11 The term “dietary supplement” refers to “a product (other than tobacco) intended to supplement the diet that bears or contains one or more of the following dietary ingredients: a vitamin; a mineral; an herb or other botanical; an amino acid; a dietary substance for use by man to supplement the diet by increasing the total dietary intake.”12 The FDA and various state and local drug regulatory authorities and academic researchers frequently find that dietary supplements that are sold in U.S. stores or are available through the Internet are contaminated with heavy metals13 or adulterated with prescription medications.14 For example, PC-SPES was an herbal product manufactured and distributed by a U.S.-based company and advertised as preserving prostate health, but it was extensively used by patients with prostate cancer. PC-SPES was also being investigated as a potential prostate cancer therapy until the product was voluntarily withdrawn from the market in 2005 after the California Department of Health Services and the FDA published warnings indicating that several production lots were found to contain various amounts of warfarin and diethylstilbesterol.15 Adverse effects of a dietary supplement may be due to the labeled contents in the supplement or a contaminant or adulterant. In the case of herbal supplements, instances have occurred of the substitution of a different herb for one listed on the label, sometimes because of misidentification of the plant. The Natural Medicine Comprehensive Database16 and Micromedex17 contain referenced information about the adverse effects of a large number of dietary supplement components. The following sections provide examples of some adverse effects that are relevant to patients with cancer. The leaves of chaparral, a desert shrub also known as the creosote bush, are reportedly used in Native American medicine.18 The plant contains nordihydroguaiaretic acid, a compound that is under investigation as an anticancer agent.19 Renal and hepatic toxicity have been associated with chronic use of products that contain chaparral.22–22 Kava is an herbal remedy generally recommended for the treatment of anxiety or to improve sleep.23 Two randomized, controlled trials have confirmed evidence of its anxiolytic activity.24 However, several cases of severe liver toxicity associated with the consumption of products labeled as containing extracts of kava have been reported. Consequently, kava is banned in several European countries,25 and the U.S. FDA has issued a consumer advisory warning of the potential risk of hepatic toxicity from kava ingestion26; however, products containing kava may still be legally sold in the United States as dietary supplements.27 Laetrile, also known as amygdalin, is a cyanogenic glucoside found in the pits of many fruits, in raw nuts, and in other plants such as lima beans, clover, and sorghum.28,29 Although it is frequently called vitamin B17 in the lay literature, amygdalin is not recognized as a vitamin by the Committee on Nomenclature of the American Institute of Nutrition. Laetrile has been given orally and intravenously with different pharmacokinetics and toxicity profiles. Laetrile became a popular alternative cancer therapy in the 1950s and remained so through the 1980s. Currently, its sale in the United States is banned by the FDA; however, products labeled as containing laetrile can easily be purchased via the Internet. When orally ingested, laetrile can be hydrolyzed by intestinal beta-glucosidase to produce hydrogen cyanide, benzaldehyde, and glucose. The enzymatic activity of beta-glucosidase, and thus the rate of production of cyanide, can be increased under various conditions, including the presence of vitamin C.30 Signs and symptoms of cyanide poisoning have been reported both from individual patients with cancer who ingested products containing laetrile31,32 and from patients enrolled in clinical trials of oral laetrile.33,34 Cesium chloride has been promoted in books and on the Internet as a cancer therapy. No clinical trials of the use of cesium chloride have been published, but several case reports have described patients with cancer in whom QT prolongation and torsades de pointes ventricular tachycardia developed after they ingested products containing cesium chloride.35–40 In some of these reported episodes of toxicity, the patients also demonstrated hypokalemia and/or hypomagnesemia. In one case, the patient was successfully treated with 4 weeks of oral Prussian blue.35 Leaves from aloe vera, a fleshy, cactus-like plant, contain a clear gel that is often used to soothe minor skin irritations, although a systematic review of the research failed to find firm evidence for a preventive effect with regard to radiation-induced skin reactions.41 Aloe latex is an extract of the inside of the outer lining of the leaves and is approved by the German Commission E for treating constipation.42 The gel can also be made into a juice that has been promoted as a cancer cure. In a male patient with HER2+ breast cancer who was taking capecitabine, trastuzumab, and 1 L a day of aloe vera juice for 2 weeks, severe hypokalemia developed that responded to potassium supplementation after ingestion of the aloe juice was stopped.43 In clinical trials of smaller doses of aloe juice (i.e., 10 mL taken three times a day), hypokalemia has not been found to be an associated adverse effect.44,45 The root of the licorice plant has been used in TCM formulations for centuries. The now-banned herbal product PC-SPES reportedly contained Glycyrrhiza glabra,46 as do some herbal products currently used by patients with prostate cancer. Glycyrrhizic acid, a component of licorice, inhibits 11β-hydroxysteroid dehydrogenase, thus resulting in mineralocorticoid excess, which can cause hypertension and hypokalemia.47 Severe consequences of hypokalemia, including paralysis and torsades de pointes ventricular arrhythmia, have been reported in patients taking products that contain licorice.48,49 Aristolochic acid, a nephrotoxin that also may induce urothelial tumors, is found in herbs of the Aristolochiaceae family, as well as some from Bragantia and Asarum genera.50 Some of these herbs are used in TCM (e.g., guan mu tong [Caulis aristolochiae manshuriensis], which is used for dysmenorrhea and breast disorders), and they are used in some weight-loss formulas found in Europe. Case reports have been published of patients in the United States who have experienced toxicities (e.g., renal failure and urothelial cancer) after ingesting supplements derived from these plants.51 The FDA has found that some botanical products labeled as containing “Aristolochia,” “Bragantia,” or “Asarum” contain aristolochic acid.52 The enzymes of the cytochrome P450 (CYP450) superfamily are responsible for the activation or inactivation of the vast majority of drugs currently in use.53 Drugs or other exogenous chemicals ingested in the diet or via dietary supplements can affect the activity of CYP450 enzymes to a sufficient degree to meaningfully change the clinical effectiveness or toxicity of various chemotherapy drugs. Table 33-1 lists some herbs that have been shown to affect various CYP450 enzyme activities in humans and other herbs for which the effects on CYP450 enzymes have been predominantly determined by in vitro or animal studies, and thus are of uncertain clinical significance. Other herbs interfere with drug PK via effects on the activity of the P-glycoprotein multidrug transporter (e.g., curcumin) or other adenosine triphosphate–binding cassette drug transporters (e.g., genistein).54 Table 33-1 A classic example of an herb affecting drug PK is St. John’s wort. Plasma levels of the active metabolite of irinotecan, SN-38, were decreased by 42% in five patients receiving irinotecan and a commonly used dose of St. John’s wort (300 mg three times daily), apparently because of induction of the CYP3A4 isoform.55 Imatinib is also a CYP3A4 substrate, and a study using the same dosing schedule of St. John’s wort found a 43% increase in the drug’s clearance and a 30% decrease in the area under the curve of the plasma concentration.56 Green tea and its polyphenols such as epigallocatechin gallate (EGCG) can affect drug metabolism and effectiveness via actions on either PK or PD. A PD-related dietary supplement–drug interaction has been noted in preclinical studies of the combination of green tea and bortezomib. EGCG blocked bortezomib’s anticancer effect on human multiple myeloma and glioblastoma cell lines in vitro by binding to the boronic acid component of the drug; this action occurred at concentrations achievable in the blood of humans after ingesting dietary supplements containing green tea.57 In addition, the same research group reported that intragastric EGCG administration (50 mg/kg) completely abrogated bortezomib-induced apoptosis in plasmacytoma xenografts in nude mice. Another animal study also demonstrated that the antitumor activity of bortezomib on human prostate cancer xenografts in severe combined immune deficiency mice was completely eliminated with the very high plasma concentrations obtained after intravenous EGCG administration but not with the lower concentrations resulting from subcutaneous administration.58 No human studies have been performed that directly address the clinical significance of this interaction. A published case report describes a Chinese patient with metastatic renal cell carcinoma who was being treated with sunitinib and noted aggravated symptoms from a retro-orbital ocular metastasis at times when he increased the amount of green tea that he drank.59 An animal study of the effects of coadministration of sunitinib and EGCG revealed the formation of a precipitate in the stomach, resulting in lowered sunitinib absorption. Table 33-2 lists several other herb-drug interactions of potential relevance when advising and managing patients with cancer. Table 33-2 Adverse Interactions between Dietary Supplements and Cancer Therapies Several different nutrients (e.g., vitamin C, vitamin E, and selenium) and other bioactive food components (e.g., glutathione) can act as electron donors in vivo and thus are considered antioxidants. Various chemotherapy agents and radiation therapy induce the production of chemically reactive free radicals (e.g., hydrogen peroxide, ⋅OH− and superoxide, O2⋅−) that can damage a variety of cellular components (e.g., DNA and lipid membranes). Antioxidants can act as free radical scavengers and thus could potentially alter both the therapeutic and adverse effects of cancer therapies that produce free radicals. Consequently, the risk-benefit assessment for the concurrent use of high-dose antioxidant supplements during chemotherapy or radiation therapy remains controversial. Specific clinical trial results, however, have generally failed to show a detrimental effect of antioxidants on chemotherapy or radiation therapy effectiveness,60 although there is at least one notable exception. The term “vitamin E” can refer to any of eight compounds (four tocopherols and four tocotrienols) with related chemical structures and antioxidant properties, or a mixture of them. From among the individual vitamin E compounds, only alpha-tocopherol has been studied for its potential to prevent chemotherapy- or radiation therapy–induced adverse effects in humans. Alpha-tocopherol at a dose of 400 IU per day has been found to decrease the acute toxicity of radiation for patients receiving therapy for head and neck cancer.61,62 However, in the largest randomized study thus far reported, the patients who received the alpha-tocopherol supplements also experienced a higher risk of recurrence of the tumor, more frequently experienced the development of new primary tumors while receiving the vitamin E, and had a poorer mean overall survival.63,64 Various dietary supplements are suspected of having independent anticoagulant activity, although the evidence is largely from case reports. Several dietary supplements have been found to inhibit platelet aggregation to varying degrees in vitro (see Box 33-2), and human trials have demonstrated that vitamin E can augment aspirin’s antiplatelet affects.65 Table 33-3 provides some examples of supplements reported to interact with warfarin. Table 33-3 Supplements that May Interact with Warfarin Two prospective studies have addressed the potential impact of dietary modification, and to a lesser extent physical activity, on the survival of women with early-stage breast cancer. The Women’s Intervention Nutrition Study randomly assigned 2437 women with stage I to III breast cancer and who had completed their adjuvant chemotherapy, when given, to either a diet low in fat (15% of calories) and high in fruits and vegetables or a control group that received general dietary advice from a dietician.66 After a median of 60 months of follow-up, women following the low-fat diet experienced a statistically significant 24% reduced risk of relapse. The primary end point of relapse-free survival, however, was not statistically different (P = .07) for the entire study group, although women with estrogen receptor–negative tumors did have a significantly longer relapse-free survival (P = .03). Another similarly designed and sized study, the Women’s Healthy Eating and Living trial, failed to find a significant difference in recurrence rate for women on the low-fat diet, although a better recurrence rate was seen in the subgroup of women who adhered to the low-fat diet and undertook a greater degree of physical activity.67,68 Another notable difference between the two trials was that women following the low-fat diet in the Women’s Intervention Nutrition Study trial lost weight, whereas women following the investigational diet on the Women’s Healthy Eating and Living trial did not lose weight. Various foods such as legumes contain phytoestrogens; soy is particularly abundant in these compounds and has drawn the most attention regarding its potential to produce estrogenic adverse effects. Much speculation has occurred about the risk of soy intake by women with breast cancers that express estrogen receptors and, largely because of preclinical research findings about the effects of the phytoestrogens in soy, such women frequently receive recommendations to avoid eating soy products. However, an analysis of the pooled data from two large U.S. trials and one trial from China, with a total of more than 9500 women, found a nonsignificant reduced risk of breast cancer–specific mortality and a statistically significant reduced risk of breast cancer recurrence for women who consumed >10 mg of soy isoflavones per day after their cancer diagnosis. 69 Studies in which animals bearing implanted tumors are exposed to stressful conditions thought to mimic psychological stress in humans often result in increased tumor growth and metastases rates and diminished responsiveness of the tumor to chemotherapy. However, the potential for a mind-body intervention to affect the course of cancer progression or treatment response in humans has been very controversial, and few relevant experimental data are available. Several studies of support group interventions that included assessments of patient survival have been performed in patients who have cancer with conflicting results. Specifically regarding metastatic breast cancer, at least six randomized trials have been reported,70–74 although only the first study yielded positive results for survival.70 This first study found that patients who received the combined support group and hypnosis interventions experienced a longer median survival than did those in the control group.70 However, three subsequent studies utilizing a very similar intervention failed to confirm this finding, showing no benefit in survival for the patients receiving the expressive-support therapy.71,73,74 Only two studies have specifically explored the effects of psychosocial support or stress-reduction approaches on recurrence or survival in women with early-stage breast cancer. The studies utilized different support interventions, and the study that used a stress-reduction intervention (26 sessions over 12 months) found a statistically significant benefit both for risk of recurrence and survival, although the number of both events was very small.75 The other study utilized cognitive-existential group therapy weekly (20 weekly sessions) and found no effect on recurrence or survival.76 In addition to many chemotherapy agents, a few dietary compounds have been paired with gemcitabine in pancreatic cancer treatment trials. Oral curcumin at a dose of 8 g a day showed signs of single-agent activity in a phase 2 trial; however, the same dose was not as well tolerated when given with gemcitabine.81,82 A nanoparticle formulation of curcumin for intravenous administration has been produced and may prove to be better tolerated.83,84 Dr. William D. Kelley, a dentist from Texas, developed a complex regimen based on the treatment approach of an earlier alternative medicine physician, Dr. Max Gerson, and promoted it as a cancer therapy. A main component of this approach is the use of oral pancreatic enzymes, but the regimen also includes (1) vitamin and mineral supplementation, (2) concentrates of raw beef organs and glands in pill form, (3) other digestive aids, including hydrochloric acid and bile salts, and (4) a “detoxification” regimen including frequent coffee enemas.85 An initial uncontrolled study of this regimen yielded promising survival results for the subset of patients with unresectable pancreatic cancer who had the best compliance with the regimen.86 However, a subsequent prospective, nonrandomized cohort study of patients with unresectable pancreatic cancer found an inferior survival for patients who self-selected to receive the Kelly-Gonzalez therapy compared with patients treated with various chemotherapy regimens containing gemcitabine.87
Complementary and Alternative Medicine
Introduction
Nutritional Therapeutics
Dietary Supplements
Contamination and Adulteration
Adverse Effects
Chaparral (Larrea divaricata Coville)
Kava (Piper methysticum)
Laetrile
Cesium Chloride
Aloe Vera
Licorice (Glycyrrhiza species)
Herbs Containing Aristolochic Acid
Adverse Interactions between Dietary Supplements and Drugs
Cytochrome P450 Inducers and Inhibitors
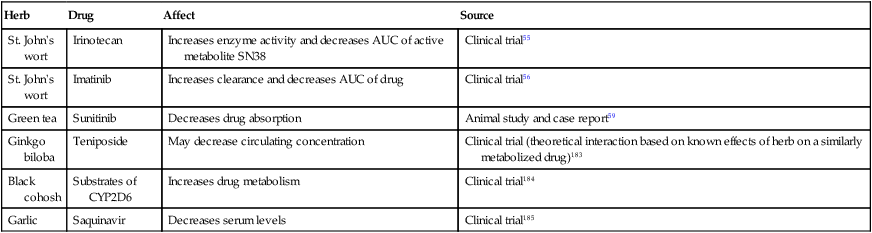
Herb
Drug
Affect
Source
St. John’s wort
Irinotecan
Increases enzyme activity and decreases AUC of active metabolite SN38
Clinical trial55
St. John’s wort
Imatinib
Increases clearance and decreases AUC of drug
Clinical trial56
Green tea
Sunitinib
Decreases drug absorption
Animal study and case report59
Ginkgo biloba
Teniposide
May decrease circulating concentration
Clinical trial (theoretical interaction based on known effects of herb on a similarly metabolized drug)183
Black cohosh
Substrates of CYP2D6
Increases drug metabolism
Clinical trial184
Garlic
Saquinavir
Decreases serum levels
Clinical trial185
St. John’s Wort
Green Tea Extract and Epigallocatechin Gallate
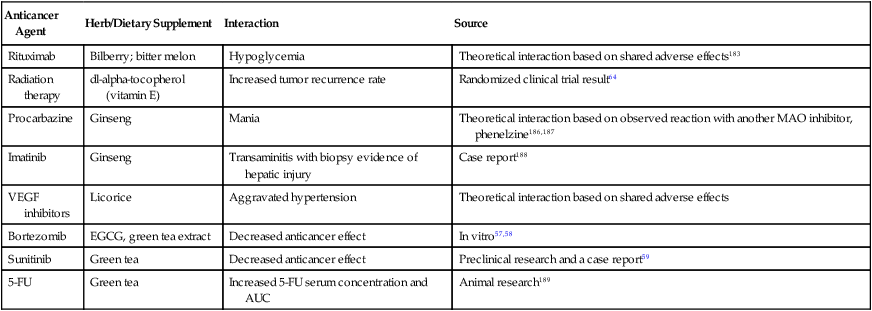
Anticancer Agent
Herb/Dietary Supplement
Interaction
Source
Rituximab
Bilberry; bitter melon
Hypoglycemia
Theoretical interaction based on shared adverse effects183
Radiation therapy
dl-alpha-tocopherol (vitamin E)
Increased tumor recurrence rate
Randomized clinical trial result64
Procarbazine
Ginseng
Mania
Theoretical interaction based on observed reaction with another MAO inhibitor, phenelzine186,187
Imatinib
Ginseng
Transaminitis with biopsy evidence of hepatic injury
Case report188
VEGF inhibitors
Licorice
Aggravated hypertension
Theoretical interaction based on shared adverse effects
Bortezomib
EGCG, green tea extract
Decreased anticancer effect
In vitro57,58
Sunitinib
Green tea
Decreased anticancer effect
Preclinical research and a case report59
5-FU
Green tea
Increased 5-FU serum concentration and AUC
Animal research189
Antioxidants
Vitamin E
Anticoagulant Interactions
Supplement
Action
Source
CoQ10
Decreased warfarin effect
Animal studies and in vitro human microsomes studies
Dan shen (Salvia miltiorrhiza)
Increased warfarin effect
Animal study
Dong quai (Angelica sinensis)
Increased warfarin effect
Animal study
Fenugreek
Increased warfarin effect
Clinical case report
Green tea
Decreased warfarin effect
Clinical case report
Cancer Treatment
Breast Cancer
Nutrition and Physical Activity
Low-Fat, High-Fruit, and Vegetable Diet
Soy
Mind-Body Approaches
Pancreatic Cancer
Curcumin
Kelley-Gonzalez Regimen
Complementary and Alternative Medicine