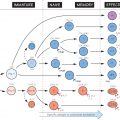
Fifteen or more serum components constitute the complement system, the sequential activation and assembly into functional units of which leads to three main effects: release of peptides active in inflammation (top right); deposition of C3b, a powerful attachment promoter (or ‘opsonin’) for phagocytosis, on cell membranes (bottom right); and membrane damage resulting in lysis (bottom left). Together these make it an important part of the defences against microorganisms. Deficiencies of some components can predispose to severe infections, particularly bacterial (see Fig. 33).
The upper half of the figure represents the serum, or ‘fluid’ phase, the lower half the cell surface, where activation (indicated by dotted haloes) and assembly largely occur. Activation of complement can be started either via adaptive or innate immune recognition. The former pathway is called ‘classic’ (because first described), and is initiated by the binding of specific antibody of the IgG or IgM class (see Fig. 14) to surface antigens (centre left); the innate, and probably earlier evolutionary pathways include the ‘alternative’ pathway, in which complement components are activated by direct interaction with polysaccharides on some microbial cell surfaces, or by a variety of pattern recognition receptors (PRRs; see Fig. 5) including ‘mannose-binding lectin (MBL) and C-reactive protein (CRP; centre left). Some of the steps are dependent on the divalent ions Ca2+ (shaded circles) or Mg2+ (black circles). A key feature of complement is that it functions via a biochemical cascade: a single activation event (whether by antibody or via innate pathways) leads to the production of many downstream events, such as deposition of C3b.
Activation is usually limited to the immediate vicinity by the very short life of the active products, and in some cases there are special inactivators (represented here by scissors). Nevertheless, excessive complement activation can cause unpleasant side-effects (see Fig. 36).
Note that, in the absence of antibody, many of the molecules that activate the complement system are carbohydrate or lipid in nature (e.g. lipopolysaccharides, mannose), suggesting that the system evolved mainly to recognize bacterial surfaces via their non-protein features. With the appearance of antibody in the vertebrates (see Fig. 46), it became possible for virtually any foreign molecule to activate the system.
Classic Pathway
For many years this was the only way in which complement was known to be activated. The essential feature is the requirement for a specific antigen–antibody interaction, leading via components C1, C2 and C4 to the formation of a ‘convertase’ which splits C3.
Ig
IgM and some subclasses of IgG (in the human, IgG1–IgG3), when bound to antigen are recognized by Clq to initiate the classic pathway.
C1
A Ca2+
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree