Fig. 11.1
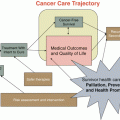
Relationships between comorbidities, breast cancer treatment and breast cancer outcomes. Comorbid conditions among breast cancer survivors can negatively influence breast cancer treatment and patient quality of life, increasing breast cancer mortality by 20–50 %, and competing cause mortality by up to sixfold. The large influence of comorbidities on competing cause mortality suggests that early stage breast cancer patients are more likely to die from competing causes than from breast cancer, and that addressing comorbid conditions effectively will be important to further gains in improving breast cancer prognosis. Research is needed to understand how effective control of comorbid conditions, as an interplay of management approach, choice of medications used, and patient adherence to treatment of comorbid conditions, affects breast cancer treatment, quality of life, breast cancer prognosis, and competing cause mortality, and reciprocally, how a diagnosis of breast cancer affects comorbid conditions, their management, and control. The influence of specific comorbidities and various management approaches used to treat these conditions, including choice of medications used, also needs to be evaluated. These questions are particularly important for African Americans, older women, and women of lower socioeconomic status (SES) who bear higher comorbidity burdens, experience worse breast cancer prognosis, and potentially utilize health care differently compared to the general population. Studies aimed at understanding the pattern of comorbid conditions among breast cancer patients, the larger set of lifestyle and biological risk factors contributing to these conditions, and their impact on quality-of-life and competing cause mortality in these women will be strengthened by including comparison samples of women without a history of breast cancer with similar burdens of comorbidity. This knowledge, particularly the interplay of factors that contribute to the various breast cancer outcomes, and how relationships between breast cancer treatment, quality-of-life, breast cancer prognosis, and competing risk mortality are potentially modified by comorbidity and their management will be instrumental in reducing disparities in disease prognosis experienced by breast cancer patients with comorbidities
Current Research
Comorbidities and Survival Outcomes
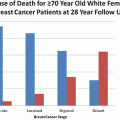
There is ample evidence indicating that breast cancer patients with comorbidities have poorer overall disease prognosis, which includes increased breast cancer specific deaths as well as death from other causes (Sogaard et al. 2013; Edwards et al. 2013; Patnaik et al. 2011b; Land et al. 2012a, b; Berglund et al. 2012; Bush et al. 2011). In a US population of older breast cancer patients, women with stage 1 tumor and a comorbid condition had similar or poorer overall survival compared with patients with stage 2 cancer and no comorbid conditions (Patnaik et al. 2011a). In a cohort of ~125,000 breast cancer patients aged 65 years of age or older, diagnosed between 1992 and 2005 residing in 11 Surveillance, Epidemiology, and End Results (SEER) areas, the prevalence of 16 comorbidities that contribute to the Charlson Comorbidity Index was considerable with 32.2 % of women having 1 or more conditions, similar to the prevalence (31.8 %) observed among women without breast cancer receiving Medicare benefits (Edwards et al. 2013). Most women with 1 or more comorbid condition fell into the severe comorbidity group, which referred to illnesses, such as congestive heart failure and chronic renal failure that often led to organ failure or systemic dysfunction requiring adjustments in cancer treatment. Moderate comorbidity referred to conditions such as diabetes and vascular disease that sometimes required modifying cancer treatment, while low comorbidity referred to conditions that usually did not require adjustments to cancer treatment. Among women aged 66–74 years in this cohort, the probability of dying from breast cancer among those with severe comorbidities was twofold higher (6 % vs 3 %) than in women without any comorbidities if diagnosed with local cancer, and 37 % higher (20.2 % vs 14.7 %) if diagnosed with regional cancer. The probability of dying from other causes in this same group of women was substantially raised, with probabilities of dying from other causes observed at 23.3 %, 10.6 %, and 5.1 % among women with severe, low/moderate, and no comorbidities, respectively. As expected, women diagnosed with distant stage disease were most likely to die of their breast cancers (≥69 %) regardless of their comorbidity status, although non-breast cancer related deaths still accounted for 5 to 20 % of deaths in this group. These findings reported in the 2014 Annual Report to the Nation on the Status of Cancer (Edwards et al. 2013) underscore previous findings that breast cancer patients with one or more comorbidities are at substantially increased risk of death from competing causes and at modestly increased risk of breast cancer specific death (Sogaard et al. 2013; Patnaik et al. 2011b; Land et al. 2012a, b; Berglund et al. 2012; Bush et al. 2011), and that breast cancer survivors who are most likely to be impacted by comorbidities are women with early stage breast cancer who have high other cause mortality, who have shown little or modest improvements in breast cancer specific mortality over time (Edwards et al. 2013; Cronin-Fenton et al. 2007; Land et al. 2012a; Izano et al. 2014). Findings from over 15 retrospective cohort studies (Cronin-Fenton et al. 2007; Tammemagi et al. 2005; Yancik et al. 2001a; Schonberg et al. 2010; Carlsen et al. 2008; Dalton et al. 2007; Janssen-Heijnen et al. 2005; Louwman et al. 2005, Houterman et al. 2004; Nagel et al. 2004; Maskarinec et al. 2003; Du et al. 2008; Harris et al. 2008; McPherson et al. 2002; Siegelmann-Danieli et al. 2006) suggest that comorbidity increases risk of competing cause mortality by up to sixfold, while breast cancer specific mortality is increased by 20–50 % (Patnaik et al. 2011a; Berglund et al. 2012; Schonberg et al. 2010; Dalton et al. 2007; Du et al. 2008), although some studies have failed to observe differences in breast cancer recurrence or survival (Tammemagi et al. 2005; Braithwaite et al. 2012; Field et al. 2011).
The larger influence of comorbidities on competing cause mortality compared to breast cancer mortality suggests that most early stage breast cancer patients with comorbidities will die from competing causes before they die of breast cancer, and that the former is a larger contributor to the disparities in overall mortality observed among these women (Ring et al. 2011). This understanding points to the overall importance of addressing comorbid conditions appropriately if further gains are to be made in improving overall survival among breast cancer patients. To date, most studies examining breast cancer outcomes have focused on breast cancer related deaths to the exclusion of competing causes of deaths. Given that most breast cancer patients are diagnosed at an early stage disease due to better screening efforts, and are expected to have good disease prognosis due to advancements in breast cancer treatments, greater understanding of factors contributing to high competing-cause mortality in breast cancer patients needs to become an important research priority (Cho et al. 2013). It should be noted, however, that although comorbidities in early stage breast cancer patients contributes to higher mortality rates among breast cancer survivors, these women are no more likely than the general United States population to die from other conditions (Cho et al. 2013). This comparison, however, may mask the true impact of comorbidities among early stage breast cancer patients (who often find their cancers through screening), since these women may be more likely to engage in healthy behaviors, have higher socioeconomic status, and show greater access to health care, including routine physician monitoring for existing comorbid conditions compared to the general population (Cho et al. 2013; Bush et al. 2011). Even among older women in the SEER-Medicare database, women diagnosed with DCIS or stage 1 breast cancer have slightly lower mortality than non-cancer controls (Schonberg et al. 2011). Early stage breast cancer patients should instead be compared to patients without breast cancer who participate in breast cancer screening programs to determine if early stage breast cancer patients are more likely to die from other conditions compared to a similar group of women without breast cancer. If death rates are found to be higher, this might suggest that further improvements to overall mortality in these patients can be made by reducing deaths due to other causes among women with early stage disease.The potential long-term impact of comorbidities in combination with a diagnosis of breast cancer on non-breast cancer deaths are also not well understood in relation to the general population. Very few prior studies include comparisons to women without a history of breast cancer (Ording et al. 2013; Cho et al. 2013; Schonberg et al. 2011; Snyder et al. 2013; Hanchate et al. 2010), and virtually none have matched on comorbidity status to help understand potential differences in the long-term impact of comorbidities in combination with a diagnosis of breast cancer in comparison to women in the general population with the same comorbidity (Ording et al. 2013). Studies including a well-characterized comparison group with extended follow-up will help to elucidate key groups at excess risk for poor outcomes among breast cancer survivors.
Effect of Comorbidities During and After Breast Cancer Treatment
Comorbidities may reduce breast cancer specific survival, in part, by reducing the likelihood that these patients receive guideline recommended treatment (Land et al. 2012b, c; Vulto et al. 2006; Jagsi et al. 2009, 2010; Bouchardy et al. 2007; Harlan et al. 2009; Ring 2010; Kimmick et al. 2014; Sabatino et al. 2014; Shayne et al. 2006), which in turn, is linked to higher rates of breast cancer recurrence (Lash et al. 2000). The impact is likely to be strongest among those with early stage breast cancer since the likelihood of a cure is highest in these women and more dependent on treatment decisions. Generally, as comorbidity increases, treatment intensity decreases, including decreased ability to complete prescribed chemotherapy treatments (Lee et al. 2011). Findings from previous studies show that breast cancer patients with comorbidities are less likely to receive surgery, axillary dissections if undergoing breast-conserving surgery, radiotherapy, and adjuvant chemotherapy (Louwman et al. 2005; Griffiths et al. 2014; Vulto et al. 2006; Jagsi et al. 2009, 2010; Harlan et al. 2009; Ring 2010; Kimmick et al. 2014; Sabatino et al. 2014; Land et al. 2012c; Shayne et al. 2006; Bouchardy et al. 2007; Velanovich et al. 2002; Stavrou et al. 2012; Dialla et al. 2012; Garg et al. 2009; Ballard-Barbash et al. 1996). Comorbidities have also been shown to predict nonadherence to tamoxifen and aromatase inhibitors (Hershman et al. 2010), although not in all studies (Hadji et al. 2013). Certain comorbidities, including congestive heart failure, chronic obstructive pulmonary disease, osteoarthritis, autoimmune disease, liver dysfunction, renal disease, and thyroid disorder can elevate risk of developing chemotherapy-induced febrile neutropenia (Chia et al. 2013; Chao et al. 2014; Hosmer et al. 2011), which can lead to chemotherapy dose delays and dose reductions (Shayne et al. 2006; Garg et al. 2009). Pre-existing comorbidities can also increase risk of treatment associated comorbidities. For instance, cardiac dysfunction, diabetes, and hypertension are all associated with greater risk of anthracycline cardiotoxicity (Lotrionte et al. 2013). An important unanswered research question is whether treatment intensity can be safely increased in those with comorbidities and by how much, and whether variations exist according to the type of comorbidity.
Guidelines for the treatment of breast cancer are mostly developed on the basis of findings from clinical trials that exclude patients with moderate and severe comorbidities, to examine the impact of breast cancer treatments without the effect of other health conditions that may interfere with treatment or increase the risk of death. These exclusions mean that participants in randomized controlled trials are generally healthier than the general population. As a result, there are limited data available on the impact of comorbidities on treatment complications among breast cancer patients and the underlying reasons for failure to complete treatment. A recent study, for instance, that evaluated the impact of self-reported comorbidities among older women receiving adjuvant chemotherapy for breast cancer while in the CALGB 49907 and CALGB 361004 clinical trials found comorbidity to be associated with shorter overall survival, but not with toxicity or time to relapse (Klepin et al. 2014), possibly because these women all had good functional status with less severe comorbidity at the time of enrollment since eligible patients could not have a medical condition that would make the protocol hazardous (Klepin et al. 2014). Greater understanding of the degree to which various comorbidities affect breast cancer treatment and ultimately breast cancer survival is a research priority, and can be addressed through both observational studies as well as randomized trials designed to more broadly examine the potential impact of new breast cancer treatments across the entire targeted patient population.
There are very limited data on the effect of breast cancer and its treatment on the development of newly diagnosed comorbidities, and whether these incident comorbidities are associated with poorer outcomes than in a comparable population without a history of breast cancer. Only a few studies have followed breast cancer patients longitudinally and assessed comorbidities at more than one time point. Harlan and colleagues (Harlan et al. 2009) reported that breast cancer patients who received chemotherapy alone, chemotherapy plus radiation or radiation plus tamoxifen were 2–3 times more likely to develop newly diagnosed comorbidities after breast cancer diagnosis than women who did not receive radiation, chemotherapy, or tamoxifen, with arthritis, hypertension and osteoporosis being among those commonly reported (Harlan et al. 2009). A study of 1,361 five year breast cancer survivors aged 65 and older compared to women without breast cancer for a 10 year follow-up period, found that comorbidities included in the Charlson Comorbidity Index were not more likely to develop in breast cancer patients compared to age-matched women free of breast cancer, although breast cancer patients were slightly more likely to die in the 10 year follow-up period beginning 5 years after diagnosis (Jordan et al. 2014), suggesting perhaps for a role for more common comorbidities not represented in the Charlson Comorbidity Index. These findings point to a need to examine the impact of comorbidities on breast cancer treatment and outcomes more broadly, beyond those represented in the commonly used Charlson Comorbidity Index.
Impact of Type of Comorbidities
To date most studies examining the link between comorbidities and breast cancer outcomes have been based on population-based cancer registry data linked with administrative health insurance claims data, with many studies taking advantage of data from the SEER-Medicare database. While these studies have been instrumental for determining the prevalence of comorbidity among older women and their impact on survival outcomes, they have largely focused on the impact of a few select comorbidities available in these databases. The most widely used of these indices is the Charlson Comorbidity Index, along with several adaptations of the Charlson Comorbidity Index, including the National Cancer Institute Comorbidity Index (Klabunde et al. 2007). A few studies have also used the Adult Comorbidities Evaluation Index (ACE-27) (Kimmick et al. 2014; Fleming et al. 2011), which considers a greater number of comorbidities than the Charlson Comorbidity Index and unlike most measures of comorbidity, considers the severity of each condition with three grades of decompensation (Kallogjeri et al. 2014). The ACE-27 method also captures obesity comorbidity, hypertension, and a wider range of cardiovascular diseases not captured by Charlson that may be particularly relevant to breast cancer outcomes. Despite differences in the number, type, and severity of comorbid conditions captured, however, the Charlson and ACE-27 indices perform similarly in predicting 2 year overall survival in cancer patients, and models including both indices produced better predictive models (Kallogjeri et al. 2014). Direct comparisons between the Charlson Comorbidity Index and ACE-27, however, have not been made for breast cancer patients and future research can be directed at testing the predictive ability of these comorbidity measures individually and together on various breast cancer outcomes.
The Charlson score was originally developed in 1987 using medical records to predict 1 year mortality among hospitalized patients, and was later shown to predict risk of death from comorbid disease in a 10 year follow-up study (Charlson et al. 1987). Reflecting the original intent of the index to predict short-term mortality, the 16 comorbidities that contribute to the Charlson Comorbidity Score tend to be more severe, requiring hospitalization, and include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease, connective tissue disease, peptic ulcer disease, type 2 diabetes, chronic renal disease, paralysis, malignant lymphoma, solid tumor, liver disease and acquired immunodeficiency syndrome (AIDS). Comorbidities are assigned a weight between 1 and 6, reflecting likelihood of dying from the disease. This was later expanded to incorporate physician claims in addition to inpatient data from Medicare files since most individuals will have comorbidities that do not require hospitalization. This allowed improved prediction of non-cancer mortality and treatment choice for breast cancer patients after development of condition weights specific for breast cancer (Klabunde et al. 2000). While the Charlson Comorbidity Index has been repeatedly shown to be a valid prognostic predictor among breast cancer patients, the index is based on underlying assumptions more relevant to short term mortality risk, with very few patients actually having a high Charlson Index score in most early stage breast cancer populations. Consequently, an important limitation of the Charlson Index is that the score tends to classify a large proportion of breast cancer patients as having no comorbid conditions. Nevertheless, the hypertension-augmented comorbidity index, an extension of the Charlson index, has been shown to be a significant predictor of overall survival, breast cancer specific, and competing cause survival in breast cancer patients (Braithwaite et al. 2009). Also, given dramatic improvements in the prognosis of individuals with AIDS in the past 20 years, the need for reappraising how AIDS is weighted in the Charlson Comorbidity Index has been raised (Zavascki and Fuchs 2007). This reassessment may be particularly important in breast cancer studies focused on women with higher relative incidence of positive HIV status and AIDS, such as young African Americans (Center for Disease Control, HIV surveillance report 2010).
Conceivably, certain comorbidities may have different effects on breast cancer treatment, quality-of-life, and survival outcomes (Louwman et al. 2005; Braithwaite et al. 2009). Very little research to date has assessed associations between individual comorbid conditions and their impact on breast cancer prognosis (Patnaik et al. 2011a, b; Yancik et al. 2001b), and whether these relationships might be modified by other prognostic factors such as estrogen receptor status or tumor subtypes. Studies that have examined specific comorbidities have largely focused on comorbidities contributing to the Charlson Comorbidity Index (Patnaik et al. 2011a, b; Yancik et al. 2001b). Patnaik et al. (2011a) showed in a large cohort of >63,000 breast cancer cases, using SEER-Medicare linked data, that breast cancer patients with any of the comorbidities comprising the Charlson Comorbidity Index had lower survival rates compared to patients with no comorbidities, and that liver disease, chronic renal failure, dementia, and congestive heart failure were associated with the highest all-cause mortality, while cardiovascular disease, COPD, and diabetes, specifically raised breast cancer deaths by 10–25 % (Patnaik et al. 2011a, b), presumably due to less intensive treatment and/or to direct biologic effects, e.g. diabetes is associated with reduced likelihood of receiving chemotherapy and increased glucose and insulin which have been associated with poorer outcomes (Goodwin et al. 2012; Gold et al. 2014; Peairs et al. 2011).
Generally, cancer patients who report more comorbid conditions report lower quality of life, including poorer physical and mental health (Smith et al. 2008). Specific comorbidities have been shown to increase adverse effects of breast cancer therapy although most of this research has focused on the effects of obesity and related cardiovascular risk factors (Schmitz et al. 2013). Diabetes for instance is a risk factor for paclitaxel neuropathy and increased risk of neuropathic pain following breast surgery (Lee and Swain 2006; Wilson et al. 2013). Hypertension and obesity are risk factors for development of heart failure with trastuzumab, and development of postoperative lymphedema, fatigue and worse functional health (Schmitz et al. 2013; Helyer et al. 2010; Perez et al. 2008).
The effects of specific comorbid conditions on various breast cancer endpoints, including quality of life, needs to be further examined, although, going forward, comorbidities considered should be expanded beyond those in the Charlson Comorbidity Index to include major chronic health conditions that are highly prevalent in the United States’ population (US Burden of Disease Collaborators 2013) and among breast cancer patients (Piccirillo et al. 2008; Sarfati et al. 2013), including obesity, high blood pressure, diabetes, metabolic syndrome, cardiovascular disease, respiratory disease, and psychiatric diseases. Such research may be aided by recent development of the Chronic Condition Warehouse (Centers for Medicare and Medicaid Services, Chronic Condition Warehouse, accessed 10/11/14), which combines Medicare, Medicaid, and Part D Prescription Drug Events data and makes these datasets available for research. The Chronic Conditions Warehouse was designed to support studies on improving care for chronically ill beneficiaries and contains 27 annual chronic condition flags indicating the presence of specific diagnostic codes on Medicare claims. These include chronic conditions such as asthma, anemia, depression, Alzheimer’s, hyperlipidemia, osteoporosis, arthritis, and diabetes. Understanding which comorbid conditions have the greatest impact on breast cancer specific outcomes may provide insights into common etiological risk factors shared between the comorbidity and breast cancer, and suggest improved management strategies that offer the best gains in disease outcomes.
Use of Cancer Registry and Administrative Claims Data to Study Comorbidities
While use of cancer registry and administrative claims data has helped to define the link between comorbidities and breast cancer outcomes at the population level, the scope of questions that can be posed in studying the effects of comorbidity on breast cancer is constrained by a number of limitations inherent in these databases (Riley 2009). Presently cancer registries do not routinely collect data on comorbidities, although for some populations, these data can be obtained by linking with Medicare or Medicaid data, hence the popularity of using linked SEER-Medicare data. Secondly, as discussed above, a very limited number of comorbid conditions are usually considered and many of the more common, minor, chronic conditions are not assessed. Development of the Chronic Condition Warehouse linking Medicare and Medicaid data, however, can help facilitate the study of how common chronic conditions impact cancer outcomes. Other challenges include examining the impact of disease duration and severity, which is difficult to gauge because changes in claims for a specific comorbidity may be a function of payment rules rather than variability of the comorbidity over time. Incident disease in claims databases are hard to identify, and limited availability of clinical information in these databases means that the underlying reason for service and outcomes are unavailable. Some conditions, particularly less severe ones, tend to be under-diagnosed and under-reported in insurance claim data, and comorbidities might be missed if only inpatient care is considered. These include conditions such as osteoporosis, dementia, arthritis, and low back pain, which are usually treated in outpatient settings, are not associated with short-term mortality, and often do not require hospitalization. Patient movement in and out of insurance claims databases may also limit the utility of these data for prospective comorbidity studies, making populations who do not have continuous health coverage difficult to study.
If using Medicare data, one problem with studying women close to age 65 will be that these women have less “at risk” time to appear in Medicare claims. One study found that 12 % of people enrolled in Medicare at age 65 waited more than 2 years before making their first use of Part B services, which includes medically necessary services and preventive services (Sloan et al. 2012). Use of SEER-Medicare data also excludes examination of comorbidities among women who are diagnosed with breast cancer at younger ages, who are more likely to have aggressive estrogen receptor negative breast cancers. SEER areas are also known to have lower proportions of Caucasians, to be more urbanized, and to have fewer people living in poverty, which may make findings less generalizable (Warren et al. 2002).
Future studies on the impact of comorbidities on breast cancer treatment and outcomes will need to use study approaches that complement findings obtained by studying large administrative databases, which have not been able to provide data on the impact of comorbidities on treatment delivery, complications, toxicities, and patient tolerance of treatments, nor on quality-of-life in these women. Some of these questions can only be answered with prospective studies of breast cancer patients. Findings from these studies will be able to provide information on the duration and severity of comorbidities that affect cancer treatment and outcomes, and how these relationships are potentially modified by management and control of coexisting conditions. Such studies will also be able to address potential confounders, including functional status and lifestyle factors such as smoking, diet, and physical activity that are not available in most administrative databases. Thus to improve research on the impact of comorbidity on breast cancer outcomes, an expansion of study approach is needed along with collection of information from a greater number of data sources. This includes the use of survey data, administrative data, detailed clinical data, prescription records, and patient medical records from all health care providers.
Research Priorities
Management and Control of Comorbidities and Impact on Breast Cancer Outcomes
The majority of breast cancer patients have at least one chronic disease condition at the time of diagnosis, but management of these conditions may be overlooked during survivorship care, leading to poorer outcomes (Weaver et al. 2013). Consequently, an important research priority will be to determine whether adequate management and control of comorbid conditions among breast cancer patients is associated with greater likelihood of receiving guideline-recommended breast cancer treatment, better quality-of-life, and better survival outcomes, including better breast cancer survival as well as competing cause survival. Studies, including those using a randomized clinical trial design, are needed to assess the importance of primary care physician involvement in the care of breast cancer patients with co-morbidities to ensure that co-morbidities are optimally diagnosed and managed, and to facilitate collaborative care between the oncologist and primary care provider (Oeffinger and McCabe 2006) as an essential component of survivorship planning identified in the 2005 Institute of Medicine report From Cancer Patient to Cancer Survivor: Lost in Transition (Hewitt et al. 2005). Studies using administrative data suggest that breast cancer survivors who see both their oncologist and primary care providers are more likely to receive preventive health services such as cholesterol screening, mammograms, and flu vaccination (Snyder et al. 2009), and breast cancer patients who have 5–10 primary care physician visits in the 2 year period prior to their breast cancer diagnosis have lower breast cancer mortality and all-cause mortality compared to those who had 0 or 1 primary care physician visit, which was only partly explained by greater use of screening mammography (Fisher et al. 2013).
The management and control of traditional risk factors for cardiovascular disease and their impact on breast cancer outcomes will be particularly important to understand since individuals diagnosed with early stage breast cancer will more often die of cardiovascular disease than from breast cancer recurrence (Patnaik et al. 2011b; Weaver et al. 2013), and cardiovascular risk factors, including obesity, hypertension, and diabetes are more common among breast cancer survivors than the general population (Weaver et al. 2013). These comorbidities may be particularly important among African American women, who have high rates of obesity, hypertension and diabetes, and may account for some of the survival disparity observed between African American and Caucasian women (Tammemagi et al. 2005; Braithwaite et al. 2009; Polednak 2004).
A related research priority will be to understand how breast cancer impacts the care and control of comorbid conditions, which may include increasing non-adherence to chronic disease medications. In a study of 1,393 women with breast cancers who were also statin users (Calip et al. 2013), the percent of women who were adherent with statin use was 67 % prior to breast cancer diagnosis, fell to 52 % during the breast cancer treatment period, and remained low in the years that followed breast cancer treatment. Similarly, the percent of women adherent with use of oral type 2 diabetes medications declined from 75 % prior to breast cancer diagnosis to 25 % during breast cancer treatment, and rose up to 32 % three years post treatment, but never returned to baseline levels. This coincided with declines in glycemic control, with the proportion of women with percent glycosylated hemoglobin levels (HbA1C) ≤ 7 dropping from 65 % in the year prior to diagnosis to 52 % during treatment and 45 % three years post-treatment (Calip et al. 2014). Compared to adherent users during the breast cancer treatment period, non-adherent users of oral diabetes medications tended to have higher stage breast cancers, were more likely to have been treated with chemotherapy, and were more likely to have ≤1 visit to their primary care provider within the year following breast cancer diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree