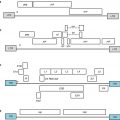
Cytokine
Condition
Chemotherapy
Phase
State
Reference
Outcome
IL-2
Melanoma
Dacarbazine
II
Ongoing
NCT00553618
Melanoma
+Cy
II
Ongoing
NCT01833767
Breast cancer
+Paclitaxel
II
Ongoing
NCT01134250
Breast cancer
+Doxorubicin
II
Ongoing
NCT01131364
Pancreatic cancer
+Gemcitabine
I
Ongoing
NCT01198522
IL-2+ IFN-α
RCC
5FU + gemcitabine
II
Completed
NCT00003664
Melanoma
Cisplatin + dacarbazine + vinblastine
III
Completed
NCT00002882
IL-15
Metastatic melanoma
+Cy + TILs
I
Ongoing
NCT01369888
Skin cancer
+Flu + TILs
II
Ongoing
NCT01369888
IFN-α
RCC
+Vinblastine
III
Completed
72
Increased RRa similar OSa
RCC
+Cis-retinoic acid
III
Completed
73
Similar RRa Similar OSa
RCC
+Cis-retinoic acid
II/III
Completed
74
Increased OSa
RCC
+5FU
II
Completed
76
No additional side effects
HCC
+5FU + cisplatin
II
Completed
80
No additional side effects similar OSa
Ovarian carcinoma
+Carboplatin/paclitaxel
III
Completed
NCT00047632
Glioma
+Temozolamide
III
Ongoing
NCT01765088
GI, renal, and lung cancer
+5FU
II
Ongoing
NCT01658813
GM-CSF
Breast cancer
+FLAC
I
Completed
NCT00001269
Finally, different forms of immunotherapy including cytokines should be investigated for overall clinical benefits along with conventional chemotherapy in patients at early stages of the disease such as after surgical resection with increased likelihood of recurrence. Further research is required to optimize the combination of different immunotherapy plus chemotherapy to obtain maximal clinical benefit.
11.6 Concluding Remarks
Combined immunotherapy clinical trials in cancer patients are challenging, and several strategies have been opened for clinical applications. However, the high efficacy of different immunotherapeutic strategies at eliminating tumors in animal models is in contrast with the very limited results achieved in patients. There are many explanations to why immunotherapeutic strategies fail or have little impact on patient survival. In general, for all solid tumors, the common scenario chosen to test immunotherapeutic protocols almost always involves patients with advanced diseases that precludes, or at least decreases, the possibility of success. Then, due to the advanced status of the cancer, the immune system of the majority of treated patients is deteriorated and unable to recognize tumor antigens. Thus, conventional chemotherapy could act in synergy to generate immunity against many tumors. The different forms of immunotherapy including the use of cytokines should be tested for overall clinical benefits along with conventional treatment regimens evidencing improvement in survival.
References
1.
Woude GF, Kelloff GJ, Ruddon RW, Koo HM, Sigman CC, Barrett JC, et al. Reanalysis of cancer drugs: old drugs, new tricks. Clin Cancer Res. 2004;10(11):3897–907.CrossRef
2.
3.
4.
5.
Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63(15):4490–6.PubMed
6.
7.
Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137(4):1270–9.PubMedCentralPubMedCrossRef
8.
10.
Shenoy AK, Fisher RC, Butterworth EA, Pi L, Chang LJ, Appelman HD, et al. Transition from colitis to cancer: high Wnt activity sustains the tumor-initiating potential of colon cancer stem cell precursors. Cancer Res. 2012;72(19):5091–100.PubMedCentralPubMedCrossRef
11.
13.
14.
15.
Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63(7):1555–9.PubMed
16.
19.
20.
21.
Nagaraj S, Collazo M, Corzo CA, Youn JI, Ortiz M, Quiceno D, et al. Regulatory myeloid suppressor cells in health and disease. Cancer Res. 2009;69(19):7503–6.PubMedCentralPubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree