Combination chemoradiotherapy achieves excellent results for the treatment of localized Hodgkin lymphoma. However, late toxic effects occur, mostly related to the radiotherapy administered after the standard adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) chemotherapy. The most serious sequelae are radiation-induced secondary cancers. Reducing radiotherapy has not yet prevented late malignancies. However, when radiotherapy was omitted, tumor control was inferior, with more relapses necessitating rescue treatment including high-dose chemotherapy with stem cell support. Early fluorodeoxyglucose positron emission tomography performed after a few cycles of ABVD is evaluated in several randomized trials to identify patients who might be safely treated with chemotherapy alone.
Key points
- •
Combined modality treatment is standard of care in localized Hodgkin lymphoma (HL) and results in an excellent cure rate but with an excess of late toxicities related to radiotherapy.
- •
Most of the localized patients can be cured with chemotherapy (CT) alone, but omitting radiotherapy, even in highly selected interim fluorodeoxyglucose positron emission tomography–negative patients, reduces tumor control and induces more relapse, some of them, although not all, being rescued with high-dose CT and autologous stem cell transplantation.
- •
The challenge is to evaluate if this excess of relapse equals or exceeds the rate of late serious effect.
- •
It is our goal to move toward this more individualized treatment and, possibly one day, the treatment of patients with HL will use newer drugs and be decided according to individual characteristics.
Introduction
Decades of Successes
The treatment of patients with Hodgkin lymphoma (HL) is one of the major success stories in oncology, and 70% to 90% of patients are cured of their lymphoma, depending on clinical stage and risk factors. Radiotherapy (RT) was used to cure patients with HL very early after its discovery, but more successes came in the 1960s for early-stage diseases IA and IIA with the use of extended field RT techniques to include all nodal stations above the diaphragm, such as the mantle field. The addition of chemotherapy (CT) to radiation improved the cure rate, and combined modality treatment (CMT) of CT followed by localized RT became the standard treatment of patients with early-stage HL.
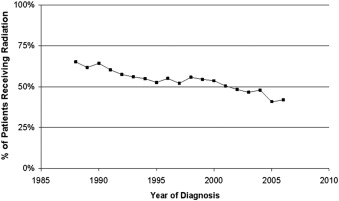
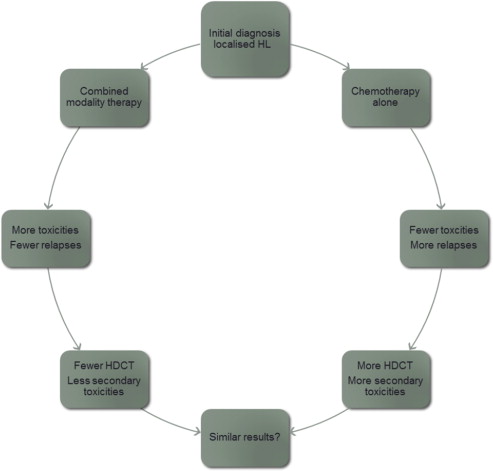
However, from the long follow-up that was available for these young patients, it seems that the price to pay for these successes was late toxicities, mainly secondary malignancies. CT was implicated in these secondary cancers (SCs) earlier as nitrogen mustard, vincristine, prednisone, and procarbazine (MOPP)-induced secondary leukemias. However, this CT has no longer been used since the CALGB (Cancer and Leukemia Group B) trial, which showed that it was less effective and more toxic than adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD). Most of the secondary malignancies are related to RT, because they occur within the field of irradiation. Other late toxicities such as cardiovascular events are related to both CT (anthracyclines) and RT. Although RT has evolved during the last decades by using lower doses and especially smaller fields, there is still a continuous trend to reducing its use ( Fig. 1 ). Several investigators have therefore proposed treating patients with localized HL with CT alone, because most of them can be cured with this treatment. However, the price to pay for this result is an excess of relapses of the HL seen in nearly all trials. The paradigm in patients with HL is that even patients in relapse can be rescued by high-dose CT (HDCT) with autologous stem cell transplantation (ASCT) and have a chance for secondary cure. The 2 proposed pathways for reaching a cure in localized HL are summarized in Fig. 2 . Which of these pathways leads to fewer toxicities overall is the subject of considerable and sometimes furious debate and is the subject of this article, which focuses on a combination of CT and RT in localized HL.
Introduction
Decades of Successes
The treatment of patients with Hodgkin lymphoma (HL) is one of the major success stories in oncology, and 70% to 90% of patients are cured of their lymphoma, depending on clinical stage and risk factors. Radiotherapy (RT) was used to cure patients with HL very early after its discovery, but more successes came in the 1960s for early-stage diseases IA and IIA with the use of extended field RT techniques to include all nodal stations above the diaphragm, such as the mantle field. The addition of chemotherapy (CT) to radiation improved the cure rate, and combined modality treatment (CMT) of CT followed by localized RT became the standard treatment of patients with early-stage HL.
However, from the long follow-up that was available for these young patients, it seems that the price to pay for these successes was late toxicities, mainly secondary malignancies. CT was implicated in these secondary cancers (SCs) earlier as nitrogen mustard, vincristine, prednisone, and procarbazine (MOPP)-induced secondary leukemias. However, this CT has no longer been used since the CALGB (Cancer and Leukemia Group B) trial, which showed that it was less effective and more toxic than adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD). Most of the secondary malignancies are related to RT, because they occur within the field of irradiation. Other late toxicities such as cardiovascular events are related to both CT (anthracyclines) and RT. Although RT has evolved during the last decades by using lower doses and especially smaller fields, there is still a continuous trend to reducing its use ( Fig. 1 ). Several investigators have therefore proposed treating patients with localized HL with CT alone, because most of them can be cured with this treatment. However, the price to pay for this result is an excess of relapses of the HL seen in nearly all trials. The paradigm in patients with HL is that even patients in relapse can be rescued by high-dose CT (HDCT) with autologous stem cell transplantation (ASCT) and have a chance for secondary cure. The 2 proposed pathways for reaching a cure in localized HL are summarized in Fig. 2 . Which of these pathways leads to fewer toxicities overall is the subject of considerable and sometimes furious debate and is the subject of this article, which focuses on a combination of CT and RT in localized HL.
Background
Treatment
RT alone is not an option
A few years after the discovery of radiographs, at the beginning of the twentieth century, HL was shown to be very sensitive to this radiation. With improvements in irradiation techniques, localized HL became curable with radiation therapy alone. When CT developed in the 1940s and after, HL proved to be a chemosensitive cancer. The introduction of newer agents and their combination into programs, such as MOPP and ABVD by investigators, including DeVita and colleages and Bonadonna and colleagues, proved that HL could be cured even in advanced stages.
Several randomized trials have compared RT alone (subtotal nodal irradiation [STNI]) with CT plus RT (CMT) for patients with localized tumors ( Table 1 ). The addition of CT to STNI gives even better results in terms of event-free survival (EFS), and this became the standard of care. RT alone was no longer an option. However, as the follow-up of these young patients extended, it seems that several late toxicities were associated with this success.
| Trial | Treatment | Number of Patients | Outcome (%) | Survival (%) |
|---|---|---|---|---|
| GHSG HD7 | A:2 ABVD + EFRT 30G | 312 | 91 | 94 |
| B:EFRT 30G | 305 | 75 ( P <.001) | 94 | |
| SWOG #9133 | A:3AV + STLI | 165 | 94 | 98 |
| B:STLI | 161 | 81 ( P <.001) | 96 | |
| EORTC/GELA H7 F | A:6EBVP + IFRT | 168 | 90 | 98 |
| B:STNI | 168 | 81 ( P <.001) | 95 |
SCs and Late Toxicities
Evaluating the late impact of our treatments
The improved prognosis of HL has been accompanied by increased risks of SCs, cardiovascular disease, and infections. Several risk factors associated with late complications were established, which are summarized in Table 2 . Treatments are being adapted based on increased knowledge of treatment-related morbidity and mortality. However, evidence concerning late toxicities suffers from several imperfections, making interpretation of the data difficult.
| Breast Cancer | |
| Evolution of risk | Increasing after 10 y |
| Risk factors | Female gender |
| Age at diagnosis (<25–30 y) | |
| RT (dose, volume) | |
| Protecting factor | Premature menopause (alkylating agents) |
| Lung Cancer | |
| Evolution of risk | More premature if the patient is older at diagnosis |
| Risk factors | Thoracic RT (dose dependent) |
| Alkylating agents (dose dependent) | |
| Tobacco use | |
| Cardiovascular | |
| Evolution of risk | Increase after 5–10 y |
| Risk factors | Thoracic RT (dose dependent) |
| Anthracyclines (dose dependent: doxorubicin: >200–300 mg/m 2 ) | |
| Classic cardiovascular risks (eg, diabetes, hypertension) | |
| Thyroid | |
| Evolution of risk | Continuous (peak at 3–5 y) |
| 50% during first 10–20 y, half of them during the first 5 y | |
| Risk factors | RT |
First, late effects occur after long and even very long follow-up. Treatments for which long-term follow-up data are available are often no longer the standard of care. Franklin and colleagues described a good example of that situation in a meta-analysis. RT as a first-line treatment strategy for stage I–III patients leads to a higher overall rate of all SC and non-Hodgkin lymphoma (NHL) than a combined modality strategy. RT alone is no longer an option, because more patients relapse compared with CMT. In the same meta-analysis, no difference in SC rates was seen between RT and CMT if follow-up times were censored at HL progression/relapse. This finding suggests that the excess SC risk after RT may be caused by the greater progression/relapse rate and therefore greater need for intensive salvage therapy.
Second, the greatest source of uncertainty may be the reliability of reporting of SC. In particular, the earlier trials were not designed to provide information on SC risk; underreporting is likely. Most trials assessed their SC information as probably incomplete. Few had cross-checked with death or cancer registries. Comparison of observed SC rates with those expected from cancer registry data implied serious underreporting in a few trials. On the other hand, SC rates may be overreported, in the sense that patients without an event are more likely to be lost to follow-up. A bias in the estimation of treatment effect would result only if such reporting biases differed between treatment arms. Furthermore, some trials did not record SC.
The number of events related to late effects is small, and therefore, large cohorts of patients need to be recruited to analyze late toxicities. In most cases, several trials are pooled or meta-analysis is performed, leading to a strong heterogeneity in the analyzed population.
Caveats in interpreting clinical trials for localized HL
Absence of Evidence is Not Always Evidence of Absence
Concerning clinical trials for localized HL, several points should be emphasized to allow correct interpretation of the results.
First, patients with localized HL is generally classified in 2 categories: favorable and unfavorable. The prognostic factors used to define these 2 groups are different around the world ( Table 3 ). Therefore, the results obtained for favorable patients by 1 collaborative group might not be automatically adaptable to other groups. Although some differences are minor (eg, 3 vs 4 lymph node areas), this situation is unique in lymphoma clinical research. A global consensus should certainly be reached but has not yet been obtained. Moreover, some groups like the German Hodgkin Study Group (GHSG) will include nodular lymphocyte predominant HL in their trials and others (Lymphoma Study Association [LYSA], Italian Lymphoma Foundation [FIL], European Organization for Research and Treatment of Cancer [EORTC]) will not.
| EORTC/LYSA | GHSG | Canada | ||
|---|---|---|---|---|
| RFs | A | Mediastinal mass | Mediastinal mass | |
| B | Age ≥50 y | Extranodal site E | Age >40 y | |
| C | ESR ≥50 | ESR ≥50 | ESR >50 y | |
| D | ≥4 nodal areas | ≥3 nodal areas | ≥3 sites | |
| E | B+ ESR >30 | |||
| Stage | ||||
| Favorable | I–II without RF | I–II without RF | I–II without RF | |
| Unfavorable | I–II with RF | I–IIA with 1 or + RF | I–II with RF | |
| IIB with C/D without A/B | ||||
| Advanced | III–IV | IIB with A/B without C/D | III–IV | |
| III–IV | ||||
Second, the primary end point of the trial should be carefully identified. This primary end point will influence the statistical design of the trial and the number of events needed to answer the question. Currently, the results reached for HL are excellent. There are, therefore, few events (relapse/refractory patients or death related to the treatment) and even fewer patients dying from the disease, because patients can be rescued by HDCT and ASCT and be cured. The primary end point of studies is therefore generally progression-free survival (PFS), and many patients are needed to answer a question in a phase 3 trial. For trials with PFS as their primary end point, the absence of difference in overall survival (OS) should not be overinterpreted. The absence of evidence for a difference cannot be translated into an evidence of absence of difference, because it generally reflects the lack of statistical power: the trial was not designed to show a difference in OS. However, a few trials will have OS as their end point. In a survival trial, the definition of an event (death) is simple. but the number of patients should be dramatically increased or the follow-up needs to be very long. With very long follow-up, additional patients will die from late toxicities and increase the number of events. The risk associated with very long follow-up is that at the time the expected number of events is reached and the data are mature, the standard of care (standard arm) may have changed and the data of the trial are no longer relevant.
Third, the type of trial must be considered. On one hand, in superiority trials, the experimental arm is expected to be superior to the standard arm. However, the results in localized HL are excellent, and further improvements will be small, at the price of many randomized patients. On the other hand, a reduction in the toxicity of the treatment might be the goal, and then a noninferiority trial is proposed. In this kind of trial, the experimental arm is supposed to be less toxic but nearly as effective. Usually, a slightly lower efficacy of the less toxic experimental treatment has to be accepted. The bigger the tolerated difference, the lower the number of patients needed. However, it is difficult to precisely define what is an acceptable difference with a previous standard. Would we allow a 15% difference in the PFS because we know that a lot of patients can be rescued by HDCT and ASCT? Or will we accept only 5%, because we do not want to submit our patients to intensive toxic therapies? This almost philosophic debate remains controversial.
Because the number of events is small in our trials, a few unrelated events (suicide, car accident, unexplained death) might lead to differences that confuse the interpretation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree