CLASSIFICATION AND HISTOLOGIC FEATURES
The classification of these lesions has varied among different authors. We currently categorize them as columnar cell change, columnar cell hyperplasia, or flat epithelial atypia (FEA).
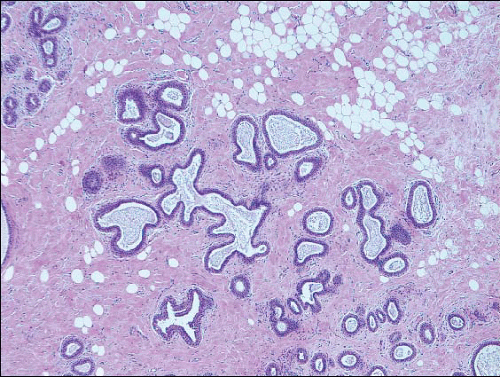
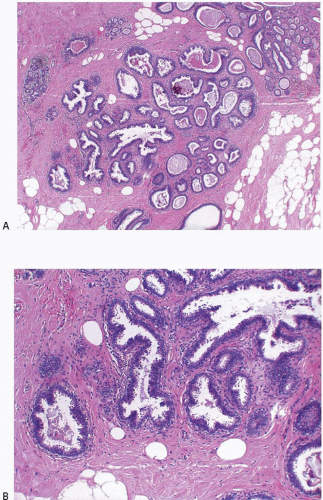
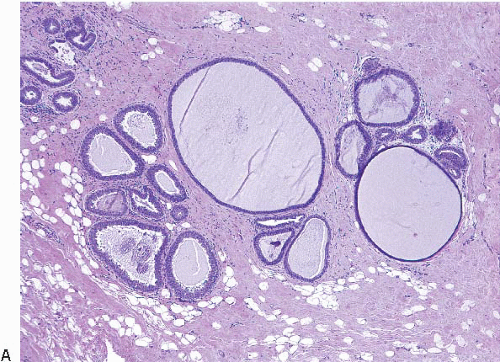
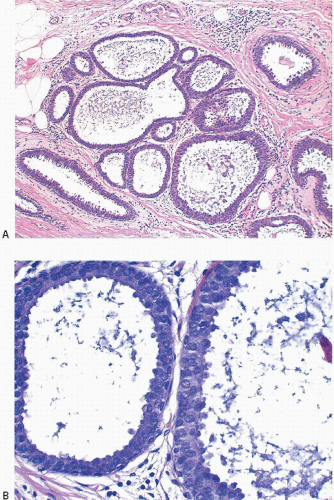
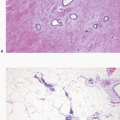
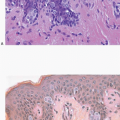
2Columnar cell change is characterized by enlarged TDLUs with variably dilated acini that often have an irregular contour (
Fig. 4.1,
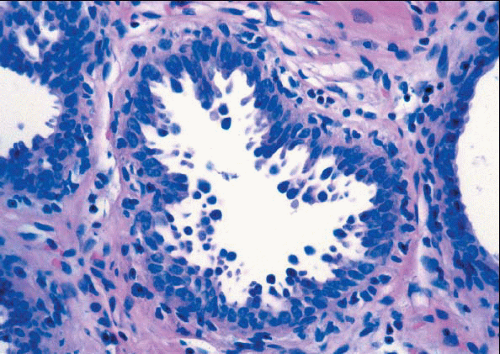
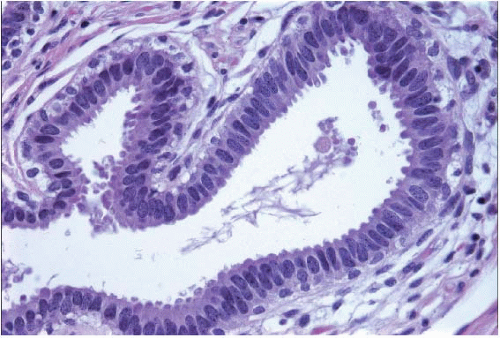
e-Fig. 4.1). The acini are lined by one or two layers of columnar epithelial cells with uniform, ovoid to elongated nuclei oriented in a regular fashion perpendicular to the basement membrane, with evenly dispersed chromatin and without conspicuous nucleoli (
Fig. 4.2,
e-Figs. 4.2,
4.3,
4.4,
4.5 and
4.6). Mitotic figures are rarely encountered. Apical cytoplasmic blebs or snouts are often present at the luminal surface of the epithelial cells but are not usually prominent or exaggerated. Flocculent secretions may be present in the lumina of the involved acini. In addition, luminal calcifications may be present.
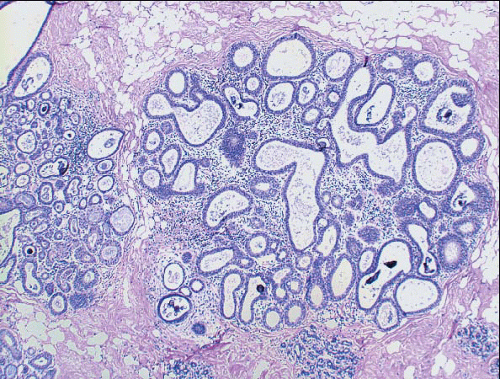
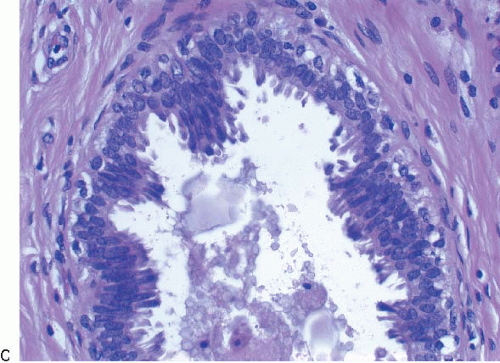
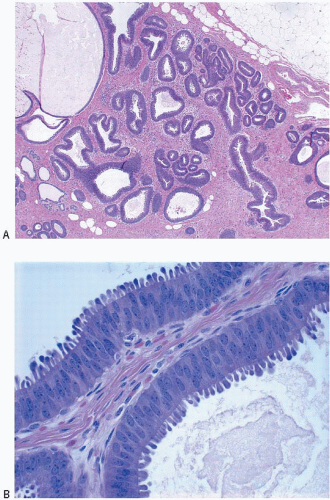
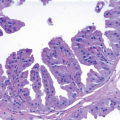
Columnar cell hyperplasia similarly features enlarged TDLUs with variably dilated acini, which are often irregular in contour. These acini are lined by columnar cells that have cytologic features similar to those seen in columnar cell change but that, in addition, show cellular stratification of more than two cell layers. Again, the nuclei are ovoid to elongated and, for the most part, oriented perpendicular to the basement membrane. Crowding or overlapping of the nuclei in these proliferative foci may give the appearance of nuclear hyperchromasia. The proliferating columnar cells may form small mounds, tufts, or abortive micropapillations (
Fig. 4.3,
e-Figs. 4.7,
4.8,
4.9,
4.10 and
4.11). Exaggerated apical cytoplasmic snouts and abundant flocculent intraluminal secretions are often present, and some of the cells comprising such lesions may have a hobnail appearance (
Fig. 4.4). These lesions frequently show intraluminal calcifications, which in some instances may have the configuration of psammoma bodies.
Lesions that we now categorize as columnar cell change and columnar cell hyperplasia have been previously described under a variety of other names, including atypical lobules type A, columnar alteration of lobules, columnar metaplasia, blunt duct adenosis, enlarged lobular units with columnar alteration, hyperplastic unfolded lobules, hyperplastic enlarged lobular units, and columnar alteration with prominent apical snouts and secretions without atypia.
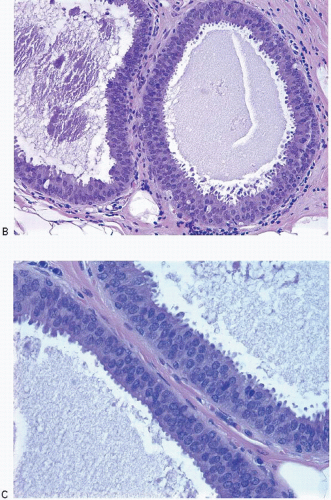
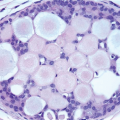
5FEA consists of enlarged TDLUs in which the native epithelial cells are replaced by one to several layers of cuboidal to columnar epithelial cells that show cytologic atypia of the low-grade or monomorphic type.
6 The acini of the involved TDLUs are variably dilated and often have round contours. The cytologic atypia of FEA is characterized by the presence of relatively monomorphic, round to ovoid nuclei that resemble those seen in the cells comprising low-grade ductal carcinoma in situ (DCIS). These nuclei are not regularly oriented perpendicular to the basement membrane and show an increase in the nuclear/cytoplasmic ratio (
Figs. 4.5 and
4.6,
e-Figs. 4.12,
4.13,
4.14 and
4.15). As a result of this increased nuclear/cytoplasmic ratio, the involved TDLUs typically have a more basophilic appearance at scanning magnification than normal TDLUs. Cellular and nuclear stratification are seen in some cases. The nuclear chromatin may be evenly dispersed or slightly marginated and nucleoli are variably prominent. Mitotic figures may be seen, but are uncommon. In some cases, apical cytoplasmic snouts or blebs may be prominent or exaggerated, and the cells cytologically may resemble those comprising the tubules of tubular carcinoma. In a minority of cases of FEA, the nuclei retain a more oval shape as well as an orientation perpendicular to the basement membrane (
Fig. 4.7). However, in contrast to the relatively slender, bland nuclei of columnar cell change and columnar cell hyperplasia, the chromatin in these nuclei may show clumping and margination, nucleoli are variably prominent, and the nuclear/cytoplasmic ratio of the cells is markedly increased.
The epithelial cells in FEA lesions may form small mounds, tufts, or short, abortive micropapillations. However, complex architectural patterns such as well-developed, club-shaped micropapillations, rigid cellular bridges, bars and arcades, or sieve-like fenestrations are not present nor is there evidence of cellular polarization within the micropapillations and bars or
around the fenestrations. Thus, it should be apparent that
flat is a relative term and simply denotes the absence of the complex architectural patterns described previously. These lesions also frequently show intraluminal calcifications, which in some instances may have the configuration of psammoma bodies (
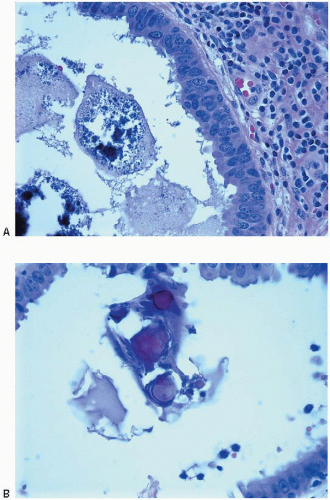
Fig. 4.8). However, the mammographic appearance of the calcifications associated with FEA is non-specific.
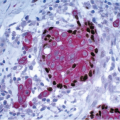
7 A variable lymphocytic infiltrate may be present in the stroma surrounding spaces involved by FEA (
Fig. 4.9). Lesions currently included within the category of FEA have previously been described by a wide assortment of other names, most notably “clinging carcinoma” of the monomorphic type (
Table 4.1).
8,
9 and
10Columnar cell change, columnar cell hyperplasia, and FEA may coexist in the same breast and even within the same TDLU. Therefore, these diagnoses should not be considered mutually exclusive. The histologic features of these lesions are summarized in
Table 4.2. An algorithmic approach to the diagnosis of columnar cell lesions and FEA is presented in
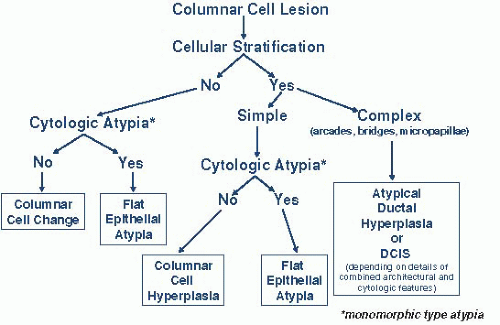
Figure 4.10.