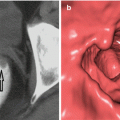
1. Neoplastic polyps
Benign (adenoma)
Tubular adenoma
Tubulovillous adenoma
Villous adenoma
Malignant (carcinoma)
Noninvasive carcinoma
Carcinoma in situ
Intramucosal carcinoma
Invasive carcinoma (through muscularis mucosa)
2. Nonneoplastic polyps
Hyperplastic polyp (including serrated polyps)
Mucosal polyp (normal mucosa in a polypoid configuration)
Juvenile polyp (retention polyp)
Peutz-Jeghers polyp
Inflammatory polyp
3. Submucosal lesions
Colitis cystica profunda
Pneumatosis cystoides coli
Lymphoid polyps (benign and malignant)
Lipoma
Carcinoid
Metastatic neoplasms
Other rare lesions
Neoplastic Colon Polyps
Adenomas
Two-thirds of colon polyps are adenomatous polyps. Adenomatous polyps are more common in men than in women, and mostly located in the left colon. The importance of these polyps is that they may show malignant degeneration; however, it does not mean that all colorectal adenomatous polyps will turn into cancer.
Adenomatous polyps are classified according to the histological structure of the predominant glandular pattern, and almost all of them contain dysplasia of different degree. According to the World Health Organization, adenomas are classified as tubular if at least 80 % of the glands are of the branching, tubule type, and as villous if at least 80 % of the glands are villiform [3]. Out of all adenomatous polyps, tubular adenomas account for 80–86 %, tubulovillous for 8–16 %, and villous adenomas for 3–16 % [4, 5].
The malignant potential of adenomas depends on size, histological type, and degree of dysplasia. Larger adenomas with a villous component and a higher degree of dysplasia have more malignant potential (Table 9.2).
Flat adenomas were first defined by Muto et al. [8]. Macroscopically, they are flat or depressed in the middle. In recent years, they attract more attention due to their potential for malignancy and the fact that they may easily be overlooked. This potential risk has prompted researchers, particularly in Japan, to adapt better methods of detection, involving dye spraying (chromoendoscopy) to generate a contrast relief-map image of the mucosa, or magnification colonoscopy, for enhanced visualization [9, 10].
Diminutive polyps are 5 mm or less in diameter. In previous years, it was thought that almost all of the diminutive polyps were nonneoplastic; it was found in recent studies that about 30–50 % of diminutive polyps were adenomatous [11–13].
Risk Factors
The frequency of adenomatous polyps shows social differentiation, but the primary factor that determines the prevalence is age. In studies conducted in the USA, the polyp prevalence was found to be about 50 % in autopsies performed on individuals over 70, and 25–40 % in colonoscopy scans performed on asymptomatic individuals over 50 [14]. Diet-related factors are also risk factors in adenoma progression. The lack of consumption of fruits and vegetables, low-pulp and fat-rich diet, low folate intake, excessive alcohol consumption, and smoking are factors that increase the risk of adenomatous polyp progression. While physical inactivity increases the risk of adenoma, aspirin and nonsteroid anti-inflammatory drugs reduce adenoma frequency. Genetic risk factor has been clearly identified in hereditary polyposis and hereditary nonpolyposis syndromes. There are also observations that suggest the presence of genetic risk factors in sporadic cases. First-degree relatives of patients with colorectal adenoma have 2–3 times increased risk.
There is at least one lesion (synchronous) simultaneously detected at a different location in 30–50 % of cases with adenoma; if there are two or more synchronous lesions, the risk of metachronous lesion or cancer progression in the following 6 months and thereafter increases accordingly.
An association between progression of colorectal adenomatous polyps and some extra intestinal diseases was reported. There is an increase in the frequency of colon cancer and colon adenomas in acromegaly [15]. Bacteremia and endocarditis caused by S. bovis have been associated with colorectal carcinoma, adenomatous polyps [16, 17]. There is a positive correlation between atherosclerosis and adenomatous polyps and colon cancer [18]. While an increase in frequency of colorectal cancer in patients with cholecystectomy was reported in some studies [19], any increase in frequency of colon cancer or adenoma was not found in general case-controlled studies [20].
Clinical Features
Most patients with colonic polyps have no symptoms referable to the gastrointestinal tract. If there is a symptom, the most common presenting symptom of colorectal polyps is occult or overt rectal bleeding. Constipation, diarrhea, and abdominal distention are the other symptoms of colorectal polyps.
In colorectal adenomas, the risk of carcinoma is 2.5 % in 5 years, 8 % in 10 years, and 24 % in 20 years after the diagnosis for polyps 1 cm in diameter [21].
Diagnosis
Colon polyps are usually asymptomatic and often detected in colonoscopic screening for colon cancer. In asymptomatic cases, the fecal occult blood test is not sufficient since blood positivity can only be detected 20–40 % in adenomas and 5–10 % in carcinomas. Small adenomas mostly do not bleed and adenoma was detected in colon in 32–41 % of fecal occult blood negative cases. Radiologically, double-contrast colonography can be used to detect colorectal adenomas; however, polyps may be overlooked due to anatomical variations and indirect nature of colonography [22]. Colonography is insufficient especially in detecting diminutive polyps. The most reliable method for detecting colorectal polyps is endoscopic diagnostic techniques. The first 25 cm from the anal canal can be examined by rigid rectosigmoidoscopy, and about 60 cm of the area up to left splenic flexure can be examined by flexible sigmoidoscopy. Considering that polyps are mostly localized in the left colon, these examinations make it possible to detect polyps at about 50–60 %. Since detection of polyps in the left colon will require searching a synchronous lesion in other parts of the colon, pancolonoscopic examination should be planned in these cases. The biggest advantage of colonoscopic examination is that it allows for intervention on polyps detected during the process [22].
Examination performed by converting three-dimensional images obtained with spiral CT into endoscopic image of colon mucosa is called “virtual colonoscopy.” It can be used as an alternative diagnostic and scanning method in selected cases because it is noninvasive and can be performed rapidly. The sensitivity and specificity is 90 % in lesions larger than 1 cm [23, 24]. Magnified colonoscopy, chromoendoscopy, and endoscopic ultrasonography are other examination methods used in the diagnosis of colorectal polyps.
Treatment
The basic treatment principle for polyps is removal. Polypectomy can be performed directly by using forceps or electrocautery snare. Endoscopic polypectomy should be performed by experienced physicians. Polyps that cannot be removed in a single session can be disintegrated and excised in a few sessions. The risk of complication is less than 2 % in polypectomies performed by experienced physicians.
Eighty to ninety percent of polyps detected in routine colonoscopy are either diminutive (≤5 mm) or small (6–9 mm) polyps. The questions regarding their removal have very important clinical consequences. However, there are differences in optimal techniques for polypectomy in this type of polyps. About 50 % of the endoscopists in America use hot or cold cautery for polyps 1–3 mm in diameter and prefer electrosurgical snare for polyps 7–9 mm in diameter. There is not an especially preferred method for polyps between 4 and 6 mm in diameter [25].
Cold forceps biopsy polypectomy is a quick, easy to apply, and cheap way. Unfortunately, this technique is associated with significant rates of incomplete polyp removal, hence, with increased polyp recurrence rates and consequent risk of interval colorectal cancer [26].
Hot forceps biopsy polypectomy was used to be very popular because it allows for simultaneous hemostasis and complete polyp excision; however, it has been pushed back because it provides bad quality biological material and gives similar results to those of cold biopsy in terms of polyp eradication [27, 28].
Cold snare polypectomy is an easy-to-apply technique that has become a favorite for small and diminutive polyps. Hot snare polypectomy is usually preferred for polyps with multiple stems [29].
In polypectomies performed by using snare, the snare which is passed through the colonoscope according to size the polyp is carefully opened in lumen. If cold cutting is planned to be performed, snare is placed around the base of the polyp and the polyp is removed. If hot polypectomy is planned, the polyp is held about 1–2 mm away from the mucosa and cauterized. Due to metallic and hard structure of the snare, it should be opened very carefully in the lumen which should be inflated sufficiently in order to prevent mucosal damage. Bleeding is more frequent in polypectomies performed with cold cutting compared to that of cauterization. Special attention should be paid to colon perforation risk in cases on which hot snare polypectomy is performed. Postpolypectomy bleedings can be intervened with adrenaline injection or using Endo Clip. Surgical follow-up and intervention is necessary for patients with colon perforation. The general approach in flat polyps is to use endoscopic mucosal resection for polyps up to 20 mm in diameter and use endoscopic submucosal dissection for polyps larger than 20 mm or giant lesions larger than 30 mm in diameter, which is a method widely used in Japan and is slowly spreading to West [30].
In cases where there is severe dysplasia, lymphatic and vascular invasion, invasion in polyp stem or bowel wall or invasive carcinoma in sessile polyp and where polyp could not be removed due to technical incompetence, surgical treatment is required [31].
Follow-Up Postpolypectomy
In line with the 2012 guidelines of American College of Gastroenterology, the patient follow-up should start with the first colonoscopic findings (Table 9.3).
Table 9.3
2012 Recommendations for surveillance and screening intervals in individuals with baseline average risk [32]
Baseline colonoscopy: most advanced finding(s) | Recommended surveillance interval (year) |
|---|---|
No polyps | 10 |
Small (<10 mm) hyperplastic polyps in rectum or sigmoid | 10 |
1–2 small (<10 mm) tubular adenomas | 5–10 |
3–10 tubular adenomas | 3 |
>10 adenomas | <3 |
One or more tubular adenomas ≥10 mm | 3 |
One or more villous adenomas | 3 |
Adenoma with high-grade dysplasia | 3 |
Serrated lesions | |
Sessile serrated polyp(s) <10 mm with no dysplasia
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|