Colorectal Cancer
Christine Parseghian
Targeting RAS
CETUXIMAB
Cetuximab is a chimeric immunoglobulin G1 (IgG1) monoclonal antibody directed against the epidermal growth factor receptor (EGFR). It does not benefit patients with metastatic colorectal cancer (mCRC) with oncogenic RAS mutations (1). The KRAS mutation was initially identified in codons 12 and 13 of exon 2, which result in constitutive activation of the RAS-RAF-MEKERK (MAPK) pathway (2,3,4). Activating mutations in KRAS are detected in approximately 40% of mCRC with good concordance between primary tumors and distant metastases of mCRC (5,6,7). Expanded RAS mutations in KRAS exon 3 or 4, or in NRAS exon 2, 3, or 4 have also been noted to predict a lack of benefit from anti-EGFR therapy (EGFRi), increasing the prevalence of all innate RAS mutations to 50% to 55% (2,8,9).
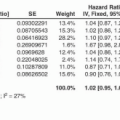
In a phase III trial of cetuximab monotherapy vs. best supportive care (BSC), patients with refractory KRAS wild type (WT) mCRC receiving cetuximab had a relative risk (RR) of 13% compared to 1% in patients with mutant KRAS (codons 12/13). Patients with KRAS WT CRC receiving cetuximab monotherapy vs. those receiving BSC experienced a longer progression-free survival (PFS) (3.7 vs. 1.9 months) and longer overall survival (OS) (9.5 vs. 4.8 months) (1). In the phase III FIRE-3 trial, KRAS/NRAS WT patients receiving FOLFIRI + cetuximab in the frontline setting experienced a RR of 72%, a PFS of 10.3 months, and an OS of 33.1 months, compared to 56%, 10.2 months, and 25 months, respectively, in KRAS/NRAS WT patients receiving FOLFIRI + bevacizumab (10).
The most significant toxicities associated with cetuximab include diarrhea, hypomagnesemia, hypocalcemia, and an acneiform rash. Traditionally, the risk of an allergic hypersensitivity reaction is reported to be <5%. However, life-threatening anaphylactic hypersensitivity reactions have been reported in up to 30% of patients residing in select geographic locations (10).
Cetuximab is currently U.S. Food and Drug Administration (FDA) approved as monotherapy for patients with mCRC who are intolerant of irinotecan-based regimens or in combination with irinotecan after progression of disease. The findings of the CRYSTAL trial also led to the approval of cetuximab in the frontline setting.
PANITUMUMAB
Panitumumab is a fully human IgG2 monoclonal antibody directed against EGFR. In a randomized phase III trial, patients with refractory metastatic disease received BSC with or without panitumumab. In KRAS WT patients, RR was 16% compared to 1% in patients with KRAS MT (mutated). In patients with KRAS, NRAS, and BRAF WT, RR was 18%. In patients with KRAS WT, but mutations in either NRAS or BRAF, the RR was 0% (11). Thus, panitumumab is now FDA approved as a single agent for patients failing irinotecan- and oxaliplatin-based chemotherapy. In another phase III trial, patients treated in the frontline setting with FOLFOX/FOLFIRI with or without panitumumab achieved an improvement in PFS and OS compared to the control arm (PFS 10 vs. 8.6 months, and OS 23.9 vs. 19.7 months) (12). This trial led to the FDA approval of panitumumab in combination with FOLFOX or FOLFIRI in the first- or second-line setting. A large randomized phase II trial (ASPECCT) (N = 1010) found that panitumumab is noninferior to cetuximab (Z score -3.19; p = 0.0007) and that these agents provide similar OS benefit in patients with chemotherapy-refractory disease (10.0 vs. 10.4 months, respectively) (13). Infusion reactions are uncommon with panitumumab because it is a fully humanized monoclonal antibody.
Targeting VEGF/VEGFR
REGORAFENIB
Regorafenib, a small-molecule inhibitor of multiple kinases that is involved with various processes including tumor growth and angiogenesis, has documented efficacy in a salvage therapy setting in mCRC. The phase III CORRECT trial randomized 760 patients who progressed on standard therapy to BSC or regorafenib. The trial met its primary endpoint of OS (6.4 vs. 5.0 months for regorafenib and placebo, respectively; hazard ratio [HR], 0.77; p = 0.005). PFS was also significantly but modestly improved (1.9 vs. 1.7 months; p < 0.000001) (14). Regorafenib is FDA approved as a salvage therapy option in patients with mCRC who have previously been treated with 5-fluorouracil (5-FU), oxaliplatin, irinotecan, a vascular endothelial growth factor (VEGF) inhibitor, and, if RAS WT, anti-EGFR therapy. It can be given before or after trifluridine-tipiracil in the third-line setting, as no data inform the best order of these therapies.
Unfortunately, although response rates to regorafenib are low, adverse events are high. The most common severe toxicities observed with regorafenib were hand-foot skin reaction, fatigue, diarrhea,
and hypertension. In a meta-analysis of four studies, the incidence of all-grade hand-foot skin reactions in 500 patients with CRC was 46.6% (15). To address this, the phase II ReDOS trial investigated the use of an alternative dosing schedule to reduce the toxicities related to regorafenib treatment. Rates of several of the most common adverse events were lower among the dose-escalation group compared to the standard dosing group. Based on these results, the dose-escalation strategy is an appropriate alternative approach for regorafenib dosing (16).
and hypertension. In a meta-analysis of four studies, the incidence of all-grade hand-foot skin reactions in 500 patients with CRC was 46.6% (15). To address this, the phase II ReDOS trial investigated the use of an alternative dosing schedule to reduce the toxicities related to regorafenib treatment. Rates of several of the most common adverse events were lower among the dose-escalation group compared to the standard dosing group. Based on these results, the dose-escalation strategy is an appropriate alternative approach for regorafenib dosing (16).
BEVACIZUMAB
Bevacizumab is a humanized monoclonal antibody that binds all isoforms of circulating VEGF, thereby inhibiting permeability and angiogenesis mediated by this factor. In a phase III trial in previously untreated mCRC, patients were randomly assigned either to receive irinotecan, bolus fluorouracil, and leucovorin (IFL) + bevacizumab or IFL + placebo. The median duration of survival was 20.3 months in the group given IFL + bevacizumab, as compared with 15.6 months in the group given IFL + placebo, corresponding to a HR for death of 0.66 (p < 0.001). The median PFS was 10.6 months in the group given IFL + bevacizumab, as compared with 6.2 months in the group given IFL + placebo. The corresponding rates of response were 45% and 35%, respectively (p = 0.004). The median duration of response (mDOR) was 10.4 months in the group given IFL + bevacizumab, as compared with 7.1 months in the group given IFL + placebo (p = 0.001). Grade 3 hypertension was more common during treatment with IFL + bevacizumab than with IFL + placebo (11.0% vs. 2.3%) but was easily managed (17). Bevacizumab is thus FDA approved for use in combination with fluorouracil-based regimens as a first- or second-line treatment for mCRC. Because bevacizumab has essentially no clinical activity as monotherapy in CRC, it cannot be recommended as a single agent and should not be considered for adjuvant therapy outside a clinical trial.
Targeting BRAF V600E Mutations
BRAF is a serine threonine kinase that is downstream of RAS in the MAPK pathway and is mutually exclusive from concurrent RAS mutations (18). The most common BRAF mutation occurs at codon 600 with a valine to glutamic acid change (c.1799T>A or p.V600E), producing a constitutively active protein (19,20). In clinical practice, approximately 5% to 8% of all mCRC carries a BRAF V600E mutation and this portends a poor prognosis (21,22,23,24). Patients with BRAF V600E mCRC have a distinct
clinical presentation accompanied with hallmark pathologic features that include older females, right-sided T4 tumors, high-grade mucinous histology, sporadic microsatellite instability-high (MSI-H) phenotype, and predominantly distal nodal involvement with peritoneal disease (25,26,27).
clinical presentation accompanied with hallmark pathologic features that include older females, right-sided T4 tumors, high-grade mucinous histology, sporadic microsatellite instability-high (MSI-H) phenotype, and predominantly distal nodal involvement with peritoneal disease (25,26,27).
In the setting of their aggressive nature and comparatively short survival in patients with BRAF V600E mutations, the initial therapeutic decisions are paramount for patients presenting with metastatic disease. There are data to support the utilization of triplet chemotherapy in combination with bevacizumab for BRAF V600E mCRC based on the TRIBE study that compared FOLFOXIRI + bevacizumab vs. FOLFIRI + bevacizumab for treatment-naïve mCRC (28). In this study, the primary endpoint of PFS was met with an improvement of 2.4 months for all patients that received the triplet regimen compared to doublet. In regard specifically to BRAF V600E, 28 patients were included in this trial, of whom 16 were randomized to the triplet arm. Subgroup analysis revealed that the triplet regimen resulted in improvement of OS to 19 months, compared to 10.7 months with the doublet regimen (28). Therefore, in the first-line setting for BRAF V600E mCRC patients with adequate performance status, FOLFOXIRI in addition to bevacizumab is a reasonable treatment choice. Of note, in this same study the RAS/RAF WT cohort that received triplet therapy had a median OS of nearly 42 months, highlighting the innate aggressiveness of BRAF V600E MT mCRC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree