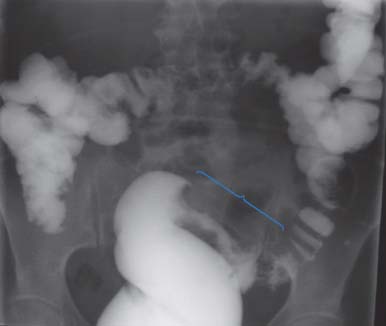
11 One of England’s greatest sportsmen, Bobby Moore died of metastatic bowel cancer in 1993 at the age of 51. He had an orchiectomy and radiotherapy for testicular cancer in 1964, two years before captaining England to World Cup victory, so although his radiotherapist may have helped win the World Cup, he may also have led ultimately to a radiation-induced secondary cancer that killed our sporting hero. This is another example of why it is better to be a medical oncologist than a radiotherapist. Colorectal cancer is a major cause of morbidity in the West. In 2011, 41,581 people were diagnosed with colorectal cancer and 15,609 died of the disease. The incidence has risen modestly over the last quarter of a century and the 5-year overall survival has doubled over the same time interval to around 50% (Table 11.1). The introduction of screening by one-off flexible colonoscopy or faecal occult blood (FOB) testing is likely to alter the incidence and mortality over the next decade. Colorectal cancers evolve over a 10-year period from polyp to invasive cancer. It is likely that a single colonoscopy every 10 years provides effective screening and prevention, but the cost of all those endoscopies for the entire population is unaffordable and so cannot be used as a screening tool. Although family history, including hereditary colorectal cancer syndromes, contributes to a third of cases of colorectal cancer, in the 1960s and 1970s, there was increasing recognition of the possibility of a dietary basis to colorectal cancer. The disease was thought to be uncommon in the developing world, whilst the high red meat/low fibre diet and obesity in the more developed market economies were seen to be responsible for a higher incidence of colorectal cancer. Much of the data on the link between diet and colorectal cancer comes from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Consumption of both red meat and processed meat (ham, bacon, sausages, pate, tinned meat) are associated with an increased risk (Table 11.2). High fibre diets increase the transit time of the stool and decrease the colorectal epithelial exposure to carcinogens within the stool. Higher consumption of dietary fibre especially cereal fibre reduces mainly left-sided colon cancers. Overall vegetable consumption also reduces the risk, but the benefit of fruit has not been confirmed in meta-analyses despite the constant advice to eat five fruits (or vegetables) a day. This advice is not based on facts and has been conjured up by some committee, in a similar way to the alcohol consumption advisory figures. Nevertheless, the authors suggest that you do not tell young children this information as the reaction of their mothers is wholly unpredictable. Table 11.1 UK cancer registration for colorectal cancer 2010 Aspirin has been shown to have a protective effect against colorectal cancer, and epidemiological studies of prolonged aspirin use have shown a consistent reduction of up to 50% in the risk of colorectal cancers. This decrease in risk is thought to be due to the inhibitory effect of aspirin on cyclooxygenase-2, which is an enzyme found in high concentrations in colorectal tissue and promotes the growth of polyps. In randomized studies, aspirin has been shown to reduce the incidence of adenomatous polyps in patients screened after the excision of a primary colorectal tumour. There has, however, only been a single randomized trial of aspirin prophylaxis, which has shown no evidence for a reduction in colorectal cancer incidence and the toxicity; especially the risk of gastrointestinal haemorrhage means that it is premature to recommend aspirin as chemoprevention. Patients with both Crohn’s disease and ulcerative colitis are at risk from developing colonic tumours, and this risk rises to over 10% after 20 years follow-up. The risk factors for colorectal cancer in patients with inflammatory bowel disease include the duration of colitis and the extent of colonic involvement. Up to 20% of patients with colorectal cancer have a family history of colorectal cancer. There are two significant familial causes for colorectal cancer: familial adenomatous polyposis (FAP) and hereditary non-polyposis colorectal cancer (HNPCC). FAP is an autosomal dominant condition that accounts for 1% of all colorectal cancer. The gene for FAP was mapped to chromosome 5q21 in 1987 and the responsible gene APC was cloned in 1991. All patients with FAP develop colorectal cancer by the age of 40 years, so prophylactic colectomy is offered to teenagers with FAP. HNPCC, or Lynch syndrome, is responsible for 2–5% of colorectal cancers. HNPCC is characterized by colorectal cancers occurring at an early age, and they are often sited on the right side of the colon. In addition, HNPCC is associated with endometrial carcinoma, and gastric, renal, ureteric and central nervous system malignancies. In this condition, the genetic abnormalities include microsatellite instability and mutated mismatch-repair genes (most frequently hMSH2 and hMLH1). In the vast majority of non-inherited colorectal malignancy, the molecular changes consist of a sequential accumulation of mutations in genes including p53 and deletion of the colorectal gene (DCC), K-Ras and APC. Indeed, it was the identification by polymerase chain reaction (PCR) of a K-Ras mutation that led pathologists to confirm the cause of death of King Ferdinand I of Aragon, the King of Naples. After his death, his body was mummified and embalmed in 1494 and placed in a wooden sarcophagus at the Abbey of San Domenico Maggiore, Naples. In 2006, an autopsy was performed revealing a large pelvic mass and the molecular analysis was consistent with a primary bowel cancer. Three-quarters of colorectal cancers are adenocarcinomas arising via an accumulation of genetic changes from benign adenomatous polyps (see Figure 2.8). Adenomas are usually slow growing and the risk of malignant transformation increases with their size and how long they have been present. Table 11.2 The effect of dietry intake on risk of colorectal cancer Figure 11.1 Medical metaphors: colon cancer: apple core lesion. Patients with colorectal tumours present to their general practitioners with a history of altered bowel habit and rectal bleeding. This may also be accompanied by weight loss and abdominal pains. These symptoms are suggestive of malignancy, and accordingly an urgent referral should be made to a specialist bowel surgeon. The patient should be seen within 2 weeks of receipt of the general practitioner’s referral letter. It is unfortunate that sometimes these symptoms are disregarded by GPs and dismissed as due to piles or irritable bowel syndrome, and IBS of course, does not cause rectal bleeding. In outpatients, the surgeon should take a full history from the patient and examine him or her. This should include a rectal examination, which may show the patient to have melaena. Proctoscopy and sigmoidoscopy should be performed in the outpatient setting. Blood tests should be organized, which should include a full blood count, renal function and liver function tests. A chest X-ray should be carried out and a barium enema or colonoscopy arranged as an outpatient procedure. The barium enema may show narrowing of the colon. In malignancy, this narrowing is typical and has the appearance of an apple core (Figures 11.1 and 11.2). Endoscopy may show a stenosing lesion or a polyp. Biopsies should be taken of the suspicious area. The tumour should be examined histologically. It is described as being either well, moderately or poorly differentiated. The original staging system for colorectal cancer was reported by Cuthbert Esquire Dukes, a British pathologist, in 1932. With various modifications this system is still in use today. Dukes’ stage reflects the degree of invasion of the tumour. Dukes’ stage A is specified when a tumour is confined to the mucosa. Dukes’ stage B is a tumour that perforates the serosa, and Dukes’ stage C is given when lymph nodes are affected. Tumours of the colon are, furthermore, divided according to their anatomical sub-sites. These are the appendix, caecum, ascending colon, hepaticflexure, transverse colon, splenic flexure, descending colon and sigmoid colon. Finally, the tumour can be staged according to the TNM clinical classification system (Table 11.3). Figure 11.2 Barium enema investigation showing irregular stricture of the sigmoid colon with shouldering giving an apple core appearance typical of sigmoid colon cancer. The suspicion of malignancy having been raised, the patient should be worked up for surgery including an assessment of operability by CT scanning and by MRI. The CT scan will show whether or not there are enlarged lymph nodes within the abdomen and will define the possibility of further spread involving the liver. The MRI is more accurate than a CT scan in defining operability and in the staging of a rectal primary. If there is no gross evidence of dissemination, the patient with a colonic tumour should be admitted to hospital for colectomy. Removal of the primary is still considered in the presence of metastatic disease to reduce the risk of perforation or obstruction. However, the use of endoscopically placed stents has developed for those unfit for surgery or as a bridge to surgery in patients presenting with bowel obstruction (Figures 11.3 and 40.13). The surgical plan depends upon the experience and practice of the clinician. There have been considerable developments in the area of laparoscopic surgery. If the patient is therefore considered to be an appropriate candidate, a laparoscopic colectomy might be performed. The results of rectal surgery are critically surgeon dependent, and much better results are obtained in centres where the surgeon specializes in this procedure. Table 11.3TNM staging of colorectal cancer Figure 11.3 How to tell small bowel obstruction from large bowel obstruction on an X-ray. At operation, a midline incision should be performed and the abdominal contents inspected. The tumour should be mobilized and removed together with a good margin of normal tissue. The tumour should be inspected and frozen sections performed to ensure that the resection edges of the apparently normal gut contain no tumour. An end-to-end anastomosis is then made. If the patient is found to have three to five liver metastases at operation, these should be resected at an appropriate time, as successful resection is associated with a good prognosis and the possibility of cure. If there are more than three to five metastases, no operative action should be taken. Extensive resection of the lymph nodes should be performed, providing histopathological information which affects the patient’s management. The surgery that is performed depends upon the site of the carcinoma and a preoperative assessment of operability. Tumours of the upper and middle third of the rectum are treated by anterior resection. In this procedure, the rectum is mobilized from the sacral hollow, and the tumour is removed together with an adequate margin of normal tissue. This normal margin ranges between 2 and 5 cm. The mesorectum and lateral pararectal tissue should be removed. Lesions of the lower third of the rectum are treated by abdominoperineal resection, which requires a permanent colostomy. The rectum is mobilized, and the peritoneum at the base of the bladder or posterior vagina is incised. The lateral ligaments are divided and the anus excised. The quality of surgery in rectal cancer is critically important. Extensive lymphadenectomy is associated with significantly improved chances for survival. A neurogenic bladder is very common after pelvic surgery but will usually recover within 10 days. Ureteric tears or transections may complicate surgery, but only rarely so. Sexual dysfunction in males is inevitable after rectal surgery, and the most common problems are retrograde ejaculation and erectile impotence. Change in sexual function in women has not been assessed because of the sexist bias of surgeons who have not thought it necessary to investigate this aspect of life. Surgery is complicated by a mortality rate of 1–2%. Following recovery from surgery, no additional treatment is recommended for patients with Dukes’ stage A disease. The value of adjuvant chemotherapy for Dukes’ B disease remains controversial. This is because no major advantage has been shown for adjuvant chemotherapy within this group of patients. Patients with Dukes’ C (node positive) tumours, however, should receive adjuvant chemotherapy. The reason for this is that there is a survival advantage in this group of patients. An online web-based tool (Adjuvant! Online) to estimate the recurrence rates, survival and benefits of adjuvant chemotherapy is available that can help patients and doctors with these decisions. The currently advocated adjuvant chemotherapy regime is FOLFOX, a combination of short-term infusional 5-fluorouracil, folinic acid and oxaliplatin administered every fortnight for 6 months. Patients with rectal cancer may receive preoperative neoadjuvant radiotherapy. This has been shown to limit pelvic recurrence for low rectal tumours. It is disputed whether neoadjuvant radiotherapy improves survival. Alternatively, after the patient has recovered from surgery, he or she may receive pelvic radiotherapy. This has been shown in randomized studies to decrease the risk of pelvic recurrence by 5–10%. Patients with more advanced tumours (T3 and T4) may be treated with neoadjuvant chemoradiotherapy prior to surgery. There is increased postoperative morbidity with chemotherapy given in conjunction with radiotherapy. Trial results show that neoadjuvant chemoradiotherapy improves disease-free survival and possible overall survival. Neoadjuvant chemoradiotherapy may lead to a complete remission in up to 70% of patients. There have been studies reported where patients achieving a complete tumour response with neoadjuvant chemoradiation have not proceeded to rectal surgery. In one of these studies just 20% of patients achieving a complete response progressed. There is a school of thought that is emerging that considers that in those patients achieving a complete response with chemoradiation observation is reasonable, reserving rectal surgery for those patients who relapse. In the situation where there are limited metastases from colorectal cancer, consideration is given to the possibility of curative surgical treatment. If the patient is fit, and there are three to five hepatic metastases or less than three pulmonary metastases, resection may be considered to be appropriate. If surgery is successful, then the prognosis is relatively good, with survival chances ranging up to 40% at 5 years. Generally, however, metastatic colorectal carcinoma has a poor prognosis, and the current recommendation for appropriate treatment is with 5FU-based regimens and radiotherapy. There is debate as to whether or not the addition of folinic acid is of an advantage to the patient. The current consensus is that there is a benefit at least in terms of remission rates, although no consensus has been reached regarding survival. The treatment regimen of first choice was called the “De Gramont regimen” and includes fortnightly 5FU and folinic acid given for 6 months. A host of new treatments have recently become available for patients with colorectal cancer. The most commonly used chemotherapy regimens are FOLFOX and FOLFIRI. The addition of irinotecan and oxaliplatin to 5FU in these regimens has improved median survival from 9 to 18 months. Recently the addition of bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor (VEGF), to both the FOLFOX and FOLFIRI regimens has led to a further modest improvement in survival. Cetuximab, a partially humanized monoclonal antibody against the epidermal growth factor receptor (EGFR), and panitumumab, a fully humanized antibody against EGFR, have both been shown to prolong survival in patients with metastatic colon cancer that lack mutations in K-Ras. Tumours are now evaluated for mutations of the Ras gene in order to define suitability for treatment. As a result of the use of these agents, survival in metastatic colorectal cancer has been extended from 18 months to almost 2 years. In this context, the cost of treatment becomes a significant political issue but, amongst the discussion on the politics of cancer care, little attention seems to be paid to the cost of not treating the patient. Dying from metastatic colorectal cancer without drug treatment is an expensive process, and the authors of this chapter are not merely considering financial cost when we make this statement. As with other cancer screening programmes, the identification of high-risk groups for screening is important. Early colonoscopic screening studies focused on very high risk groups such as those with hereditary colon cancer syndromes and those with extensive inflammatory bowel disease. It is estimated that there may be a genetic predisposition to colorectal cancer in more than 20% of patients with these tumours. In the vast majority of colorectal cases there is, however, at present no direct evidence of there being a genetic risk. Patients with a risk of developing colorectal tumours can be stratified as having low, low–moderate, moderate, moderate–high or high risk of developing malignancy. The criteria for proceeding to screening for these patients are defined as in Table 11.4. Randomized controlled studies of whole population screening with FOB have shown that screening every 2 years those aged 45–74 reduces colorectal cancer mortality by 15–18%. Conversely, a screening one-off flexible sigmoidoscopy at the age of 55–64 reduces colorectal cancer incidence by 33% and mortality by 43%. Table 11.4 Screening scheme for colorectal cancer AC, Amsterdam criteria; FAP, familial adenomatous polyposis; HNPCC, hereditary non-polyposis colorectal cancer. In 2006, the NHS bowel cancer screening programme introduced FOB tests for people aged 60–69 years every 2 years (Figure 11.4). It is estimated that if the uptake of FOB testing reaches 60% by the year 2026, 20,000 deaths from bowel cancer will be prevented in the United Kingdom. For every 1000 FOB tests completed, 20 (2%) will be abnormal and 16 (1.6%) patients will proceed to a colonoscopy, 6 (0.6%) of these will have polyps, 2 (0.2%) will have cancer and 8 (0.8%) a normal colonoscopy. The cost estimate equation for FOB testing is £1000 for each life-year saved. FOB testing will miss tumours and lead to a number of false positive findings. Colonoscopy is a more accurate means of detecting cancers but requires full bowel preparation and sedation and carries a risk of perforation (around 1 in 1500). Although the costs of colonoscopy for screening normal populations is, unfortunately, not economic, it is the investigation of choice for high-risk populations (Table 11.4). Because of studies showing the benefits and safety of one-off sigmoidoscopy, NHS England is planning to supplement FOB screening with flexible sigmoidoscopy screening of the population at 55 years of age. Figure 11.4 NHS faecal occult blood (FOB) screening kit. Case Study: A bunged up copper.
Colorectal cancer
Epidemiology
Pathogenesis
Percentage all cancer registrations
Rank of registrations
Lifetime risk of cancer
Change in ASR (2000–2010)
5-year overall survival
Female
Male
Female
Male
Female
Male
Female
Male
Female
Male
Colorectal cancer
11
14
3rd
3rd
1 in 19
1 in 14
+6%
+5%
56%
54%
Dietary factor
Effect on CRC incidence
Quantification
Meat
Red meat
Increased risk
17–30% increase in risk per 100–120 g/d
Processed meat
Increased risk
9–50% increase in risk per 25–50 g/d
Fibre
Cereal fibre
Decreased risk
10% decrease in risk per 10 g/d
Vegetables
Decreased risk
2% decrease in risk per 100 g/d
Dairy products
Decreased risk
16% decrease risk per 400 g/d 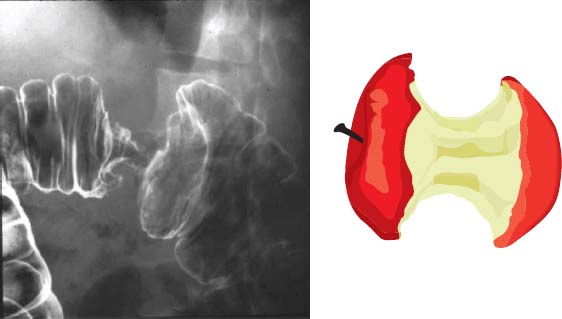
Presentation
Diagnosis
Staging and grading
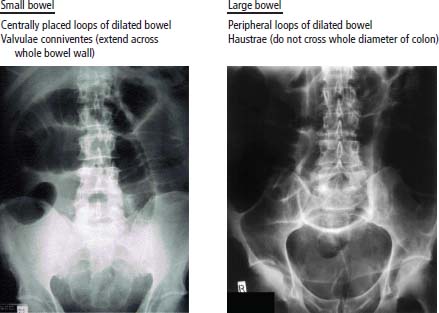
Treatment
T stage (primary tumour)
N stage (nodal status)
M stage (metastatic status)
T0: No evidence of primary tumour
N0: No nodes
M0: No distant metastases
T1: Tumour invades submucosa
N1: Metastasis in one to three pericolic nodes
M1: Distant metastases
T2: Tumour invades muscularis
N2: Metastasis in four or more pericolic nodes
T3: Tumour invades through the muscularis
N3: Metastasis in any lymph node
T4: Tumour perforates the peritoneum
Surgery for colonic cancer
Surgery for rectal cancers
Complications of surgery
Adjuvant treatment for colonic cancer
Adjuvant treatment for rectal cancer
Management of metastatic disease
Screening
Risk
Action
Age
Low risk
1 relative >45 years or
Reassure: no colonoscopy
2 relatives >70 years
Low–moderate risk
2 first-degree relatives, average age 60–70 years
Single colonoscopy
Aged 55 years
Moderate risk
2 first-degree relatives, average age 50–60 years
5-yearly colonoscopy
Aged 35–65 years or starting
1 first-degree relative <45 years
Start screening 5 years before the age when cancer was diagnosed in the youngest relative
Moderate–high risk
2 first-degree relatives, average age <50 years
3–5-yearly colonoscopy
Begin age 30–35; refer to genetics
3 close relatives (not AC)
High risk
3 close relatives AC +ve (HNPCC)
2-yearly colonoscopy
Age 25–65; refer to genetics
(FAP)
Annual sigmoidoscopy from teens
And counselling

 ONLINE RESOURCE
ONLINE RESOURCE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


