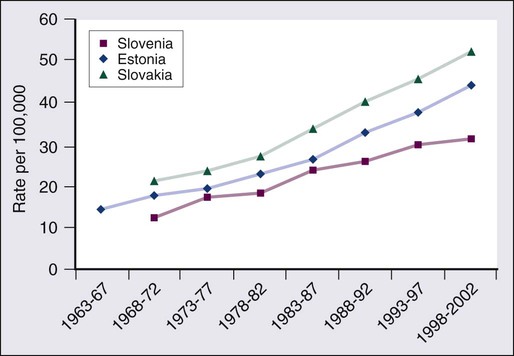
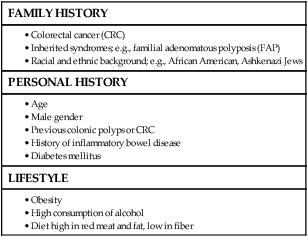
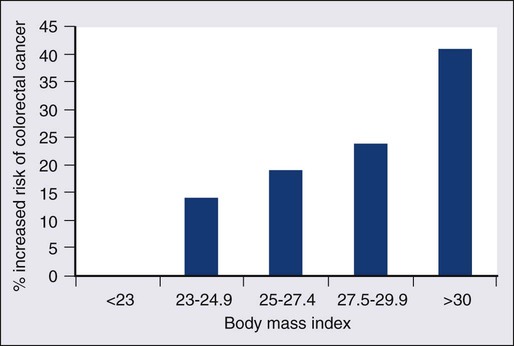
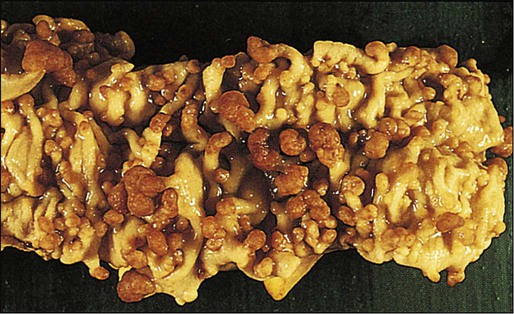
77 Sandra Van Schaeybroeck, Mark Lawler, Brian Johnston, Manuel Salto-Tellez, Jack Lee, Paula Loughlin, Richard Wilson and Patrick G. Johnston • Colorectal cancer (CRC) is the second most common cancer in women and the third most common cancer in men worldwide. • Within economically developed countries, the lifetime risk of developing CRC is 1 in 20. • Because of increased screening rates, the incidence of CRC is declining for men and women in the United States. • The incidence is 15 times higher in adults older than age 50 years, compared with those younger than age 50 years. • Inherited genetic syndromes (hereditary nonpolyposis colorectal cancer [HNPCC] and familial adenomatous polyposis [FAP]: fewer than 10% of cases) and inflammatory bowel disease (IBD) in concert with dietary and environmental exposures increases risk for CRC. • The 5-year overall survival rate has greatly improved in the last 2 decades, and is now approximately 65%, with variations across racial and ethnic subgroups. • Mortality is 35% to 40% higher in men than in women. Screening and Prevention of CRC • High level of physical activity decreases the risk of CRC by up to 50%. • Diets high in fiber and low in red, processed meat may alter risk of CRC. • Calcium/vitamin D supplementation might have preventive effects. • Aspirin and cyclooxygenase (COX)-2 inhibitors may prevent polyps and CRC, but are only recommended in high-risk patients. • Premenopausal hormone replacement therapy reduces the incidence of CRC, but increases the risk of breast cancer and cardiovascular complications. • There are mixed results in studies of the effects of statins on CRC risk. • Colonoscopy is the mainstay of screening and a useful tool in the diagnosis of CRC. Sigmoidoscopy may reduce CRC incidence and mortality. • Fecal occult blood is an acceptable screening tool. • Virtual colonoscopy, the detection of abnormal DNA within stool sample and capsule endoscopy are potentially new screening tests. • Screening is based on risk categories that take into account age; race; personal history of IBD, polyps, or cancer; family history of CRC; and presence of hereditary syndromes. • CRC is often insidious in development, underscoring the importance of screening. • Altered bowel habits, blood per anum, fatigue, anemia, and weight loss are frequent symptoms. • Obstruction is the most common acute surgical problem (approximately 30% of left-sided lesions present with an obstruction). • Approximately 5% of CRC patients will be diagnosed with synchronous cancer. The liver is the most common site for synchronous metastasis. • Approximately 20% to 40% will have synchronous polyps with cancer primary. • Computed tomography (CT) scan, magnetic resonance imaging (MRI), and positron emission tomography (PET) are imaging tools used in the staging of CRC. • Intraoperative ultrasound is the most sensitive method to evaluate liver for metastases. • Tumor size is not as critical as depth of invasion and nodal status in determining prognosis. • High histologic grade, lymphatic invasion, venous invasion, and involvement of surgical resection margins are independent adverse prognostic factors. • The “Vogelgram” highlights the involvement of specific oncogenes and tumor suppressor genes in the colorectal adenoma to carcinoma transition, and involves APC, KRAS, TP53, and DCC. • The APC tumor suppressor gene is defective in more than 80% of colon adenomatous polyps and cancers. KRAS, TP53, and BRAF are mutated in 40% to 50%, approximately 50%, and 8% of CRC, respectively. • Deletions of 18q21 (location for DCC, SMAD2 and SMAD 4) also play a role in CRC carcinogenesis. • Chromosomal instability (CIN) and microsatellite instability (MSI) pathways are closely associated to this adenoma-to-carcinoma model, and involve genes in the Wnt signaling and DNA mismatch repair pathways, respectively. • Defective DNA mismatch repair is responsible for 15% to 20% of CRC; hereditary syndromes (e.g., HNPCC); in 3% to 5% of cases, MSI positivity correlates with the inherited colon cancer HNPCC or Lynch syndrome. • Integrating genomics and transcriptomics into our standard of care remains a challenge, but is a critical component of a framework to establish a comprehensive personalized medicine strategy for the cancer patient. • The use of an enhanced recovery program (ERP) is important in the management of patients undergoing elective colonic resections. • Treatment may consist of en bloc resection of the anatomically defined portions of the colon with in-continuity draining nodes to the root of the mesocolon. • It is now recommended that, if available, a laparoscopic resection should be offered as an alternative to an open resection where both are available. • The absence of mechanical bowel preparation is not associated with a greater risk of perioperative morbidity or mortality. Curtailed carbohydrate loading, deep vein thrombosis prophylaxis, and a single dose of prophylactic antibiotics are recommended before surgery. • Experienced surgeons and the use of high-volume hospitals improve the chance of good surgical outcome. • The role of surgical resection in management of CRC metastases has led to improved outcomes for patients. • Eighty percent to 90% of recurrences are detected in the first 3 years following curative surgery. Fewer than 5% of recurrences occur after 5 years. • Many of these recurrences are solitary or limited, and when resected completely, can still cure at least 35% of patients. • A high percentage of first recurrence occurs in the liver and lung, are asymptomatic, and can be detected from a rising carcinoembryonic antigen (CEA) or CT imaging. • Of those patients who are closely followed for evidence of recurrence, 20% are candidates for salvage surgery. These patients have a 5-year disease-free survival rate of 18.6% compared with only 5.6% when metastatic disease is diagnosed because patients became symptomatic. Adjuvant Therapy in Early Stage Colorectal Cancer • Fifty percent to 60% of patients who undergo successful surgery for CRC have residual micrometastatic disease. • The goal of adjuvant therapy is to eradicate residual local disease. Recommended length for therapy is 6 months. • Combined 5-fluorouracil (5-FU) plus leucovorin regimens with oxaliplatin have further improved overall survival in patients with stage III CRC. • Adjuvant 5-FU regimens are also used in high-risk stage II CRC patients. MSI is now considered as a robust prognostic marker, in particular in stage II CRC MSI-H patients, who have a favorable clinical outcome and do not seem to benefit from adjuvant 5-FU-based chemotherapy. • In contrast to metastatic CRC, all clinical trials investigating a potential role of epidermal growth factor receptor (EGFR)- and vascular endothelial growth factor (VEGF)-targeted therapies have failed in the adjuvant setting. • Adjuvant radiation therapy has no standard role but may be considered (in conjunction with chemotherapy) in selected cases in which resection of T4 lesions leaves potentially positive circumferential resection margins. Management of Metastatic Disease • Patients with metastatic disease, in particular liver metastases, should be evaluated to determine if curative resection with or without perioperative chemotherapy treatment is possible. • Infusional 5-FU plus leucovorin with irinotecan and EGFR monoclonal antibody (mAB) cetuximab has improved overall survival to 24.9 months in patients with chemo-naive KRAS wild-type CRC tumors. • Infusional 5-FU plus leucovorin with irinotecan or oxaliplatin in combination with the VEGF mAB bevacizumab has increased median overall survival to more than 25 months. • The multikinase inhibitor regorafenib has shown to increase survival in patients who have been previously treated with 5-FU, oxaliplatin, irinotecan, anti-VEGF, and anti-EGFR agents. • KRAS is the first predictive biomarker approved for metastatic CRC. Novel adaptive clinical trial design, incorporating putative prognostic/predictive markers in prospective randomized phase II or III CRC studies, will enable a clinical validation of these biomarkers and facilitate their incorporation into routine medical practice. CRC is the second most common cancer in women and the third most common cancer in men worldwide, resulting in more than 1 million cases each year. It is expected that approximately 143,460 new cases of CRC will be diagnosed within the United States and 450,000 in Europe in 2012. In addition, with approximately 51,690 estimated deaths in 2012 in the United States and 232,000 deaths in Europe, CRC remains the second most common cause of cancer death within the Western world.1,2 Countries and regions with a western lifestyle, for example, North America, Europe, Australia, and New Zealand, have traditionally had the highest rates of CRC,3 whereas lowest rates are found in Africa and Asia.3 Within economically developed nations, the lifetime risk of developing CRC is 1/20.2 In economically transitioning countries such as in Eastern Europe, CRC incidence is increasing substantially (Fig. 77-1).4 This increase appears to reflect a change in physical activity and diet. By contrast, the United States is the only country where the incidence is declining for both males and females (Fig. 77-2). The overall incidence of CRC declined by 22% between 1975 and 2000.5 This decline has been attributed largely to increasing screening rates, which expanded from 27% to 46% of the eligible population between 1987 and 2003.6 Celebrity profile and media attention also helped increase the uptake of screening among the general population.7 In 2012, the American Cancer Society released new guidelines in relation to early detection and screening for CRC.8 The incidence of CRC is relatively rare in the under 40 years of age group and increases rapidly in older cohorts. The incidence is 15 times greater in adults older than age 50 years compared with those younger than age 50 years.9 CRC is 25% more likely to develop in men than in women, and the rate is 20% higher in African Americans compared with whites.10 The burden of CRC is likely to increase with increasing life expectancy in both males and females. Five-year survival in CRC in the United States is 65%,2 with a 26% decline in CRC-related mortality between 1975 and 2000.5 This reflects the identification and removal of (a) precancerous polyps at screening colonoscopy; (b) CRC at an earlier stage during screening (50% of reduction in mortality); (c) improved risk factor profile (33% reduction in mortality); and (d) improvements in the treatment of CRC (12% reduction in mortality). Mortality is 35% to 40% higher in men than in women. This higher rate is likely to be caused by gender-related hormone differences and gender-related risk factors. In the United Kingdom, 5-year survival rates approach 55%.11 Comparison of CRC survival rates across Europe shows significant intercountry differences.12 The majority of CRCs are sporadic, whereas a minority exhibit a familial or mendelian genetic component.13 Table 77-1 details the recognized risk factors for CRC. Certain risk factors are of sufficiently high risk to merit enhanced screening. As many as 20% of patients with CRC are likely to have a close relative with the disease and, as such, can be considered as familial in nature.13,14 Indeed, it is likely that 5% to 10% of all CRCs are syndromic. Molecular genetic testing leading to subsequent clinical management recommendations currently exists for patients with a variety of CRC syndromes, including hereditary nonpolyposis colorectal cancer (HNPCC), familial adenomatous polyposis (FAP), MYH-associated polyposis, and the hamartomatous polyposis syndromes (primarily Peutz-Jeghers syndrome, juvenile polyposis, and Cowden disease).17–17 In addition to CRC, these syndromes are accompanied by other extracolonic manifestations, some of which represent extracolonic neoplasias. The risk of CRC in an individual with a first-degree relative with CRC is increased threefold compared with those with no family history. High-risk individuals should undergo genetic counseling, with the potential for genetic testing and a program of screening by colonoscopy. Approximately 3% to 5% of CRC can be linked to HNPCC (Lynch syndrome).13 The genetic basis is a germline mutation in one of several genes involved in the mismatch repair system, most commonly hMSH2 or hMLH1, but also hPMS1, PMS2, or hMSH6. HNPCC has an autosomal dominant mode of inheritance, with less than 50% penetrance.14 Certain criteria are used to identify high-risk individuals and these are often based on the Amsterdam criteria18 and the revised Bethesda guidelines19 (Table 77-2). Lifetime risk of HNPCC is approximately 66% for men and 43% for women, with the cancer developing 25 to 30 years sooner than the median age of the general population.20 HNPCC is also associated with other cancers, in particular, of the endometrium, ovaries, and stomach. Surveillance by colonoscopy should be started by age 20 to 25 years in individuals who are mutation-proven or at risk, usually at annual intervals. In addition, mutation-proven women should have yearly screening for endometrial cancer, and prophylactic hysterectomy and salpingo-oophorectomy when childbearing is completed should be considered. In view of the strength of the clinical and molecular diagnostic techniques available, as well as a variable penetrance, it has been suggested that testing for HNPCC should be considered in all patients with CRC, regardless of their family history. Table 77-2 Criteria for Diagnosis of HNPCC • Colorectal cancer in a patient younger than age 50 years of age • A second synchronous or metachronous colorectal cancer or cancer associated with HNPCC* • Presence of high-level microsatellite instability histologically in a patient younger than 60 years of age • One or more first-degree relative with either colorectal cancer or HNPCC-associated tumor diagnosed when younger than 50 years of age • Colorectal cancer in two or more first- or second-degree relatives with HNPCC-related tumors at any age HNPCC, Hereditary nonpolyposis colorectal cancer. *HNPCC-associated tumors include endometrial, gastric, ovarian, pancreatic, ureter and renal pelvis, biliary tract, small bowel carcinoma, brain FAP is a rare autosomal dominant disorder that accounts for 1% to 2% of all CRC and is characterized by the presence of hundreds to thousands of adenomas in the colorectal mucosa by 20 to 30 years of age (Fig. 77-3).21,22 An attenuated form of FAP (AFAP) is recognized, with fewer polyps (on the order of 100 or less), and both the presentation and the development of carcinoma are delayed.23 In general, FAP is the result of an inherited defect in one allele of the APC tumor suppressor gene; however, up to 20% of cases could represent de novo mutations without an apparent family history. When the second allele is inactivated through mutation early in childhood, the colonic epithelium begins a process of uncontrolled hyperplasia and polyposis. The lifetime risk of CRC is nearly 100%, with 90% of cases occurring by 45 years of age20; lifetime risk appears to be correlated with the number of adenomas.24 In addition to the risk of CRC, other phenotypic manifestations of the disorder can include tumors of the central nervous system (also known as Turcot syndrome),25 epidermal cysts, osteomas, dental abnormalities, mesenteric or abdominal wall desmoid tumors (also known as Gardner syndrome),26 congenital hypertrophy of the retinal pigmented epithelium, and upper gastrointestinal tumors, especially periampullary duodenal carcinoma. Several of these phenotypic features have been correlated with specific mutation sites.27 This is a polyposis syndrome characterized by mutation in the MYH gene, which is involved in the DNA proofreading machinery of the genome; its failure allows for the accumulation of further mutations, particularly in the APC gene.30–30 The polyposis of this syndrome tends to be less pronounced than FAP. In addition to high-penetrance genes such as those described previously, a variety of low-penetrance polymorphic genes have been investigated for their potential to influence CRC risk, particularly when considered in the context of concomitant dietary or environmental exposures.32 Specifically, polymorphisms associated with an increased risk of CRC included the NAT2 fast phenotype (70% increased risk); the GSTT1 null genotype (40% increased risk); a number of mutant alleles of ALDH2; any of four rare variants of the protooncogene HRAS1; and the a2, a5, and a13 alleles of TNFα. More recent studies using whole-genome scanning approaches have also identified single-nucleotide polymorphisms locus associated with an increased risk of CRC at the 8q24 locus.33,34 Familial clustering of CRC is a common occurrence, even in those without a definable genetic predisposition. For example, twin studies suggest that heritable factors are important in CRC development, even in the absence of known genetic predispositions.35 Two recent meta-analyses of observational studies demonstrated an approximate relative risk of 2.25 in individuals with a single first-degree relative affected by CRC and a 4 to 4.25 relative risk in those with more than one affected relative or with a family member who developed CRC before 45 years of age.36,37 Population lifetime CRC risk estimates for a 50-year-old increased from 1.8% to 3.4% with one affected relative and to 6.9% with two or more affected relatives. Risks were also elevated approximately twofold for individuals with first-degree relatives who harbored colorectal adenomas, further supporting the thesis that colorectal adenomas and carcinomas are linked. Screening is recommended for individuals with a first-degree relative who developed CRC before the age of 50 years. Screening should commence at the age of 40 years or 10 years earlier than the earliest cancer.8,38,39 There is newer evidence that having a first-degree relative develop an advanced polyp (>1 cm or villous histology) before the age of 50 years also increases the risk of developing CRC.40 This has led some organizations (including the American College of Gastroenterology) to include it as grounds for screening of targeted individuals.8,41 The risk of CRC in individuals with a previously diagnosed adenomatous polyp is particularly increased if the polyps are large (>1 cm) or multiple (>2) or advanced (high-grade dysplasia or villous histology) and requires additional follow-up.42,43 With pancolonic inflammatory bowel disease (ulcerative colitis or Crohn disease), the risk of CRC increases with the duration of illness (2% at 10 years, 18% at 30 years).44–47 The extent of the involved colon is an important determinant of risk and no increased risk is seen when it is confined to the rectum.45 Screening is recommended to begin 10 years after the diagnosis is made and the frequency to be increased in subsequent decades or according to the degree of ongoing inflammation. There is an association between risk of developing diabetes and CRC. This is partly explained by the shared risk factors of physical inactivity and obesity (see below), but there would appear to be an additional independent risk factor.48 Obesity, particularly abdominal obesity, is a risk factor for CRC, independent of physical activity.49 The association is stronger in men than in women. Men in the highest quintile (body mass index [BMI] >30) have double the risk compared with those in the lowest quintile (BMI <23).50,51 The relationship is linear (Fig. 77-4).52 The mechanism appears to be related to insulin resistance and hyperinsulinism. An association with CRC has been demonstrated, with a relative risk of 1.26 (1.11 to 1.43) compared with nonsmokers, with the risk persisting up to 25 years after stopping smoking.54 It is estimated that 15% to 20% of CRC can be attributed to smoking,55 and the link is strongest for rectal cancer.56 Genetic studies have revealed reductions in CRC risk for polymorphisms in a number of genes, including homozygous carriers of the C677T variant allele of methylenetetrahydrofolate reductase (20% to 30% reduced risk) and heterozygous or homozygous carriers of an intron 3 polymorphism of the p53 gene.32 There is a largely consistent database to support the protective effect of physical activity. High levels of activity decrease the risk of CRC by up to 50%.57,58 This is equivalent for men and women.51,57 Even moderate physical activity (3 to 4 hours brisk walking/week) has been shown to be beneficial.58,59 Many case-control studies have shown that the high intake of dietary fiber reduces the risk of CRC. Two meta-analyses suggest a reduction of 50% in CRC risk.60,61 By contrast, prospective cohort studies have failed to show this association.64–64 Meta-analysis of these prospective cohort studies with more than 700,000 participants did indicate a modest benefit of increased fiber intake, but this association ceased to be significant when other dietary factors were taken into consideration.65 In contrast to the United States cohort studies outlined above, a European study (European Prospective Investigation into Cancer and Nutrition [EPIC]) of more than half a million participants, revealed a 40% reduction in the risk of CRC among those with the highest fiber intake.66,67 The difference between this European EPIC study and those from the United States could relate to the presence of folate fortification of food in the United States, reducing the absolute benefit of increased fiber. It is also possible that the type of fiber may be important, with unprocessed wheat bran being more effective than the processed forms.67 Resistant starches that are fermented into short-chain fatty acids in the colon, could also reduce the risk of CRC. Some studies have shown such an inverse association,68 but a randomized trial of supplementing resistant starch in European patients with HNPCC failed to note any reduction in neoplasia risk after 4 years of follow-up.69 There is a significant body of evidence supporting the relationship between red meat consumption and CRC. This is particularly true of meats that have been cooked at high temperatures for prolonged duration.57 One study demonstrated that the risk of CRC developing in a 50-year-old individual over a 10-year period was 1.7% in the group consuming the most red meat and processed meat compared with 1.3% in those in the lowest category.64,70 Both stimulation of endogenous insulin and consumption of iron or other carcinogenic factors have been suggested as the pathogenic mechanism. Several studies have demonstrated a protective role for calcium and vitamin D in risk of CRC. Pooled analyses for calcium studies revealed a 22% reduction in CRC risk in the highest quintile of calcium intake compared with the lowest quintile.71 Calcium supplementation significantly reduced adenoma recurrence rates.72 Although not proven to protect against CRC, calcium supplementation has been recommended by some national organizations. For vitamin D, a meta-analysis of prospective studies indicated a 50% reduction in CRC incidence, comparing those with the highest and lowest serum vitamin D levels.73 Although there is evidence that folate plays a protective role, trials of folate supplementation have not shown a benefit in adenoma prevention.74 This may be because of folate supplementation programs where it is added to food. One study found an increased risk of multiple adenomas with additional folate supplementation.75 Additional folate supplementation is not recommended for CRC prevention. Currently, no medications are recommended for the general population for the prevention of CRC. Cardiovascular protection trials with aspirin have demonstrated an additional benefit of reducing CRC by 50%.77,78 This effect occurred after a lag period of 5 years and was true for high-dose and low-dose aspirin. In the CAPP2 trial, aspirin demonstrated a protective effect in patients with Lynch syndrome, with a CRC incidence ratio of 0.6 (0.32 to 0.99) for those in the aspirin arm of the trial.69,79, The cyclooxygenase (COX)-2 inhibitor celecoxib and the nonsteroidal antiinflammatory drug (NSAID) sulindac have also demonstrated benefit in adenoma regression in FAP.80,81 However, complications can occur, including massive gastrointestinal bleeding or gastric ulceration. The United States Preventive Services Task Force (USPSTF) recommendations of 2007 are still valid: For average-risk individuals, the harm associated with aspirin or NSAIDs outweighs the benefit in CRC risk reduction.82 A more recent international consensus suggested that aspirin may have a role in high-risk populations.83 Similar to aspirin, statin trials for cardiovascular risk reduction have demonstrated a reduced risk of CRC.84,85 However, other case control studies have not confirmed this finding.86,87 Postmenopausal hormone replacement therapy has also been demonstrated to reduce CRC risk88; however, it is not recommended in this setting because of the associated increased breast cancer and cardiovascular disease risk.89 Three key issues for any screening program in cancer are (a) a recognizable predisease state, (b) a simple, effective, and safe test, and (c) evidence that intervention improves survival. All three of these criteria are met for CRC, which progresses from adenoma to carcinoma over a 10- to 15-year period, allowing intervention to occur either before the cancer develops or following the detection of an early, more easily treatable cancer.90 Recommendations regarding frequency of screening for high-risk individuals are contained within guidelines from national and international societies.8,38,39,91 Surveillance for these high-risk patients is via colonoscopy. The rest of this section addresses the average-risk individual and assesses the variety of screening approaches available. The three most common options for screening the average risk individual are (a) annual fecal occult blood (FOB) testing with high sensitivity tests, (b) sigmoidoscopy every 5 years (± annual FOB testing), and (c) colonoscopy every 10 years. Some national organizations have suggested a preferred screening tool, for example, the American College of Gastroenterology recommends colonoscopy.41 Others have endorsed a wider range of options, including CT colonography and fecal DNA testing.39,91 Programs vary in when they recommend commencing screening (age 50, 55, or 60 years, based on a combination of risk benefit and economic criteria). Most programs suggest stopping at 75 years of age or when life expectancy is less than 10 years. To date, the most common test has been the guaiac FOB test (gFOBt). This test is not specific for human hemoglobin, making dietary restrictions required before performing the test. The test itself has limited sensitivity when used on a one-off basis (50% for CRC). However, this increases to 90% if used repeatedly over a prolonged period.41,92 The reading and interpretation of the results are important determinants of sensitivity and specificity. The lower the threshold that labels a test as positive, the greater the number of screening colonoscopies that are required. The first randomized controlled trial of CRC screening by gFOBt was published in 1993 and demonstrated a 33% reduction in mortality among patients undertaking annual gFOBt screening over a 13-year period.93 Two subsequent randomized studies revealed similar effects. A ten-year follow-up of alternate-year gFOBt from Denmark found an 18% reduction in CRC mortality.94 A United Kingdom study, similarly performed over 10 years with testing every 2 years, revealed a 15% reduction in CRC mortality.95 A study looking at “missed” cancers that present symptomatically between the biannual FOBt demonstrated that they accounted for between 30% to 60% of the cancers and that they were more likely to occur in women and on the right side.96 That study recommended adding a one-off flexible sigmoidoscopy as an adjunct to FOB testing. Limitations of the gFOBt led to the development of a test based on immunochemistry (iFOBt, fecal immunohistochemistry test [FIT]). A randomized comparison between the two demonstrated a positivity rate of 5.5% for iFOBt that was more than double the 2.4% of gFOBt.97 This has resulted in significantly more colonoscopies and in the detection of increased numbers of cancers and advanced adenomas. iFOBt is a semiquantitative test, therefore the cutoff value can be shifted, depending on the availability of colonoscopy resources and the risk of CRC in any given population. Subject to costs, it is now the recommended methodology for fecal sampling.98 Patients with significant pathology at flexible sigmoidoscopy including high-risk adenomas (multiple, >1 cm or villous histology) proceed to full colonoscopy. There is uncertainty over the management of smaller tubular adenoma (6 to 10 mm). Most clinicians proceed to colonoscopy in these cases.41 Once-only flexible sigmoidoscopy between the ages of 55 and 64 years has been proposed as a strategy to reduce CRC incidence and mortality.99 In a randomized controlled trial, in the United Kingdom with more than 50,000 people in the intervention group, CRC incidence was reduced by 23% and mortality by 31%.100 This was after a follow-up of more than 11 years. An Italian study found an 18% reduction in incidence and a nonsignificant 22% reduction in mortality in a similar study.101 These findings have been replicated in a large Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial of more than 150,000 subjects.102 After a median follow-up of 12 years, there was a 21% reduction in the incidence of CRC, and although this reduction in incidence was seen for both right-sided and left-sided tumors, the overall 26% reduction in CRC mortality came from the reduction of mortality in left-sided CRC. Mortality from right-sided CRC was unaffected. Interim results of the Norwegian Colorectal Cancer Prevention (NORCCAP) study at 6 to 7 years (one-third of the way through the study) revealed no reduction in CRC incidence or mortality.103 Strong evidence in favor of colonoscopy comes from the U.S. National Polyp Study (NPS) which demonstrated a very significant reduction in the incidence of CRC, estimated between 76% and 90%.90 A follow-up of that study published in 2012 was also able to demonstrate a reduction in mortality estimated at 53%.104 Although these figures are based on historical comparators and expected incidence-based mortality, they do support colonoscopy as a valid screening tool. There is increasing evidence of the long-term benefits of screening sigmoidoscopy and colonoscopy. A single flexible sigmoidoscopy reduces the CRC rate for at least 11 years.100 After a negative colonoscopy, the CRC incidence was 2.8 per 100,000 in Year 1 and 13 per 100,000 of the population in Year 4 (expected rate 70.6 per 100,000).105 Most programs recommend repeating colonoscopy 10 years after a negative screening colonoscopy. Subsequent colonoscopy will be sooner in cases where polyps are found and that interval will depend on the specific findings.41,42 The lack of reduction in mortality for right-sided tumors raises questions about flexible sigmoidoscopy as a form of screening. However, there has also been a failure in the full colonoscopy studies to demonstrate any reduction in mortality for right-sided colon cancer. A Canadian case control study of colonoscopy showed the reduced cancer mortality being limited to left-sided cancers106 and this limitation has been demonstrated similarly in other studies.107,108 Several explanations have been suggested for the lack of impact of endoscopic screening on right-sided cancers (Table 77-3).109,110 The experience and skill of the endoscopist is key to the success of colonoscopy as a screening tool. In particular, a high adenoma detection rate correlates with a reduced risk of developing an interval cancer.111 Reported adenoma detection rates within the same center can vary from 9% to 33% between endoscopy specialists.112 By contrast, the mortality benefit of FOB testing is equivalent for right- and left-sided lesions.113 A key component of a successful CRC screening program is a high participation rate from the target population. The participation rate was higher for iFOBt (60%) than for gFOBt (47%).97,114 This higher uptake may be the result of requiring fewer samples, easier sample collection, and fewer dietary restrictions. In addition to lack of participation, another factor reducing the efficacy of either FOBt-based screening approach is that 16% of subjects declined follow-up colonoscopy. One recent study found lower participation rates for colonoscopy screening (38%) than for FOBt (67%).115 There was also a difference depending on the ethnic background of the population. An Italian study initially invited subjects to flexible sigmoidoscopy. Those who failed to respond were then offered iFOBt. The initial response to flexible sigmoidoscopy was between 30% and 40% and an additional 30% of the population who had failed to respond to the invitation to flexible sigmoidoscopy underwent iFOBt screening.116 This stepped approach to a screening strategy increases the population adherence to screening, and the population also benefits from reduction in CRC incidence and mortality. In 2003, the Council of Europe recommended the implementation of a population-based screening program for CRC, based on the observation from randomized trials that screening contributed to a reduction in colorectal mortality. By the end of 2007, there were screening programs in 19 of 27 European Union countries117; a number of other countries will introduce screening programs in 2012-2013. However, despite the benefits of screening, participation rates in European countries are relatively low (40% to 60%).118 All assessments of the cost-effectiveness of CRC screening have shown it to be cost-effective, and in some cases, cost-saving.119 One study comparing gFOBt, iFOBt, and colonoscopy by using accepted screening intervals, suggested that colonoscopy offered the greatest net health benefit, reducing CRC mortality by 83%, but that annual screening using iFOBt offered a better cost-effectiveness ratio of $611.00 per quality-adjusted life-year (QALY). All three of these strategies were identified as cost-effective.120 In a U.S. study looking at annual gFOBt and sigmoidoscopy every 5 years and colonoscopy every 10 years, all three were considered cost-effective.121 A review of the published literature again indicated the consistent message that CRC screening was cost-effective, but the evaluation did not show any consistency with regard to what current screening test offered the greatest cost-effectiveness.122 In addition to actual cost, the choice of screening test will also depend on the availability of resources such as colonoscopy, the general public’s acceptance of the chosen method of screening and the overall costs of the screening, which will differ between countries. Recently, there have been efforts to standardize reporting of CRC screening to facilitate comparison of programs globally.123,124 Standardization of indicators from these different programs will facilitate interprogram comparisons and help ascertain the best model for the particular circumstances of a country or region, for example, at what age should screening commence? How often should fecal testing be performed? When should any screening be discontinued? Computerized tomographic colonography (CTC) involves scanning of the abdomen and pelvis followed by reconstruction in 2D and 3D imaging to create a “virtual colonoscopy.” This technique has a high sensitivity and specificity for detecting large polyps (10 mm or larger), but a lower sensitivity for smaller polyps and does not detect flat lesions that appear to be important in right-sided CRC.125 The addition of oral contrast fecal tagging has helped to improve the interpretation of the procedure. Recognized limitations include the failure to detect smaller lesions, the exposure to ionizing radiation and the need for repeat bowel prep and colonoscopy if a lesion is found (unless the patient can move straight from radiology to endoscopy on the same day). In a study comparing CTC and computerized tomographic colonoscopy, adherence rates were higher for the CTC but computerized tomographic colonoscopy found a higher number of advanced adenomas per attendee, resulting in equivalent numbers of advanced adenomas being detected. CTC detected adenomas that required colonoscopy and overall patient experience was preferable for the direct colonoscopy.126 A second potential screening test for CRC is detection of abnormal DNA within stool samples. It is possible that this will be a future alternative to the detection of blood in stool samples. DNA testing has been improving rapidly with regard to choice of marker panel and use of buffering and preservative solutions. It has proved effective in detecting both cancers and adenoma.127 Similar to the blood-based fecal tests, DNA testing equally detects both proximal and distal neoplastic lesions.128 The greatest current drawback is cost. It has not been found to be cost-effective. However, as the technology develops and the costs per test decrease, DNA testing will emerge as another effective CRC screening tool. More detail on the potential for DNA markers is provided in the section on the molecular basis of CRC. A third suggested screening modality is capsule endoscopy.129 By swallowing the capsule endoscopy pill, noninvasive images can be obtained of the colon. However, it has not yet demonstrated sufficient sensitivity or specificity to be recommended as a screening tool. The anatomic extent of disease at presentation (stage) is the strongest predictor of survival for patients with CRC and forms the basis of appropriate patient management. The tumor, node, metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer is considered the international standard for CRC staging.130 In contrast with other staging systems for CRC, the TNM system is continuously updated on the basis of existing data, multidisciplinary in design, allows for the incorporation of all technologic approaches to staging, and has a comprehensive set of rules of application that ensure uniform use. The predictive accuracy of TNM staging can be increased through incorporation of validated prognostic features, such as lymphatic or venous invasion, into the overall assessment process. Indeed, although the basic categories for tumor direct extension, distant metastases, and lymph node involvement have not changed since the last edition, a number of new subdivisions have occurred that may have clinical significance (Table 77-4, Fig. 77-5). Table 77-4 Surgical Stage and Survival Rates in Colorectal Cancer
Colorectal Cancer
 Physician exam with CEA testing every 3 to 6 months for the first 2 years, then every 6 months until 5 years.
Physician exam with CEA testing every 3 to 6 months for the first 2 years, then every 6 months until 5 years.
 Full colonoscopy preoperatively or postoperatively, at 1 and 3 years postoperatively, and then every 3 years.
Full colonoscopy preoperatively or postoperatively, at 1 and 3 years postoperatively, and then every 3 years.
 CT scan of the thorax/abdomen/pelvis preoperatively or postoperatively, and then annually for 3 to 5 years.
CT scan of the thorax/abdomen/pelvis preoperatively or postoperatively, and then annually for 3 to 5 years.
Epidemiology of Colorectal Cancer
Incidence
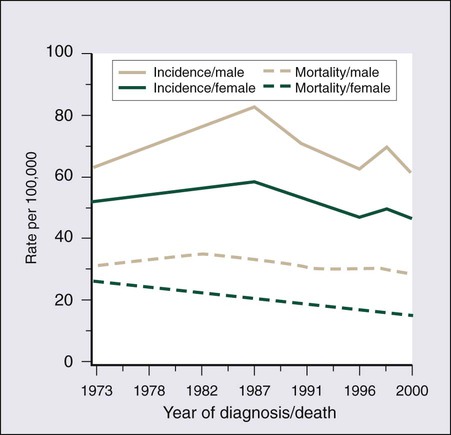
Mortality
Risk Factors for Colorectal Cancer
Inherited Colon Cancer Syndromes
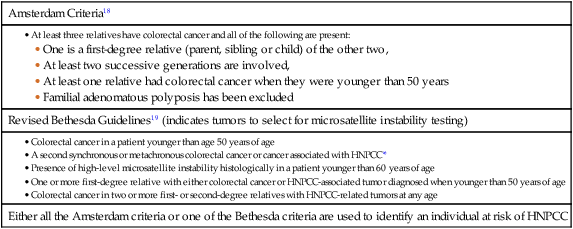
Hereditary Nonpolyposis Colon Cancer
Amsterdam Criteria18
Revised Bethesda Guidelines19 (indicates tumors to select for microsatellite instability testing)
Either all the Amsterdam criteria or one of the Bethesda criteria are used to identify an individual at risk of HNPCC
Familial Adenomatous Polyposis
MYH-Associated Polyposis
Other Genetic Factors
Family History of Colorectal Cancer or Adenomatous Polyps
Prior Polyps; Inflammatory Bowel Disease
Diabetes Mellitus; Obesity
Smoking
Screening and Prevention of Colorectal Cancer
Genetic Factors
Contribution of Physical Activity
Contribution of Diet
The Role of Dietary Fiber
Decreased Red Meat Consumption
Prevention Strategies
Calcium and Vitamin D
Folate Supplementation
Nonsteroidal Antiinflammatory Drugs, Hormone Replacement Therapy, and Statins
Screening for Colorectal Cancer
Screening Options
Screening Tests
Fecal Occult Blood Testing
Fecal Immunohistochemistry Test (FIT)
Flexible Sigmoidoscopy
Colonoscopy
Right-Sided Colon Cancers
Uptake of Colorectal Cancer Screening
Cost Effectiveness of Colorectal Cancer Screening
New Technologies in Colorectal Cancer Screening
Diagnosis and Staging of Colorectal Cancer
Diagnosis
Staging
AJCC/UICC Cancer Staging*
Comparisons
5-Year Survival Rates¶
AJCC/UICC
Tumor
Regional Lymph Nodes
Distant Metastases
Comparison Dukes†
Astler, Collier‡
SEER§
Stage 0
Tis
N0
M0
—
Limited to mucosa
In situ
Stage I
T1
N0
M0
Dukes A
Extending into submucosa
Localized
93.2
T2
N0
M0
Dukes A
Extending into muscularis propria
Localized
Stage IIA
T3
N0
M0
Dukes B
Extending through muscularis propria
Regional
84.7
Stage IIB
T4A
N0
M0
Dukes B
Extension through bowel wall
Regional
72.2
Stage IIC
T4B
N0
M0
Dukes B
Extension through bowel wall
Regional
Stage III
Any T
N1-2
M0
Dukes C
Limited to or extension through bowel wall with involved lymph nodes (LNs)
Regional
–
Stage IIIA
T1, T2
N1
M0
Dukes C
Limited to bowel wall with involved LNs
Regional
83.4
T1
N2a
M0
Dukes C
Limited to bowel wall with involved LNs
Regional
Stage IIIB
T3, T4
N1
M0
Dukes C
Extension through bowel wall with involved LNs
Regional
64.1
T2-T3
N2a
M0
Dukes C
Limited to or extension through bowel wall with involved LNs
Regional
T1-T2
N2b
M0
Dukes C
Limited to bowel wall with involved LNs
Regional
Stage IIIC
T4a
N2a
M0
Dukes C
Extension through bowel wall with involved LNs
Regional
44.3
T3-T4a
N2b
M0
Dukes C
Extension through bowel wall with involved LNs
Regional
T4b
N1-2
M0
Dukes C
Extension through bowel wall with involved LNs
Regional
Stage IV
Any T
Any N
M1
—
Distant metastases
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access