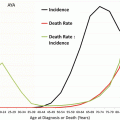
Fig. 13.1
Incidence of invasive and in situ malignancy of the colorectum (adenocarcinoma) and the anal canal (squamous cell carcinoma), United States, SEER 2000–2011. In AYAs (inset) incidence increases exponentially with age (Ries L, Bleyer A, personal communication 2015)
Incidence of CRC varies by ethnic group. In those older than 25 years, rates of invasive CRC are highest among black and non-Hispanic white populations. At ages younger than 25, rates are comparable among different races. In general, CRC in young adults is more common among minorities and in those who are uninsured [4].
As illustrated in Fig. 13.2, the incidence of CRC in general has been decreasing since 1976, likely in part related to population-based screening; however, the incidence of CRC is increasing in AYAs [2]. The cause for increasing rates of CRC in this younger population is unclear. However, the trend has been consistently demonstrated in both hospital and population-based studies [5]. It is hypothesized that there may be an association with lifestyle factors, including obesity and lack of physical activity. Although similar lifestyle trends are affecting the older adult population, older patients are benefiting from risk reduction with colonoscopy and polypectomy, in addition to other forms of screening such as fecal occult blood testing [2]. Incidence of rectal cancer in AYAs appears to be increasing more rapidly than that of colon cancer [4].
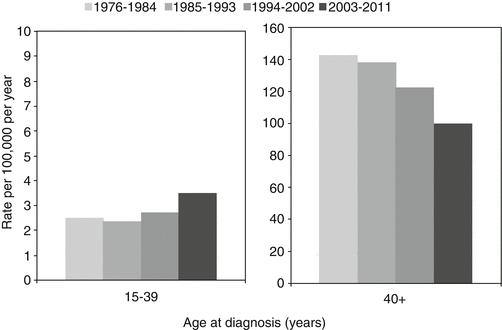
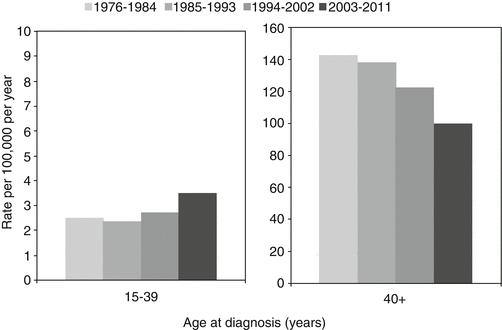
Fig. 13.2
Incidence trends for invasive adenocarcinoma of the colon by age and sex, United States, SEER 1976–2011. Rates of disease are decreasing in older adults, but increasing in AYAs (Ries L, Bleyer A, personal communication)
13.3 Etiology
13.3.1 Inherited Syndromes
The majority of cancers of the colon or rectum, even in the AYA population, are sporadic in nature. In AYAs with CRC, only 22 % of patients have a family history of colorectal cancer, and 16 % have a clearly identifiable risk factor [6]. Nevertheless, several familial syndromes are well defined and confer an increased risk of CRC. Overall, 3–5 % of CRC cases are attributable to a defined hereditary syndrome [7].
13.3.1.1 Lynch Syndrome
Lynch syndrome (LS) is the most common form of hereditary CRC. It has also been termed hereditary nonpolyposis colon cancer, named as such to distinguish it from the polyposis associated with familial adenomatous polyposis (FAP). However, this name has recently lost favor, as it minimizes the importance of non-colorectal cancer risk associated with the syndrome. In addition, individuals with LS often do develop colonic adenomatous polyps, and these polyps progress though the adenoma to carcinoma sequence much more rapidly than sporadic adenomas of the colon. Lifetime risk of CRC in Lynch syndrome is 70 % with median onset in the fifth decade [8]. Importantly, 40 % of cases are diagnosed before age 40. Lynch syndrome-associated colon cancers are often right-sided lesions with mucinous histology and are high grade. Synchronous or metachronous bowel cancers are common. There is also an increased risk of extracolonic malignancies including cancers of the endometrium, ovary, stomach, small bowel, pancreas, biliary tree, ureter, and renal pelvis [9].
The clinical diagnosis has historically been made by applying the Amsterdam criteria that rely solely on personal and family history of LS-related cancers. In the adult population, sensitivity is reported to be 78 % [10]; however, in one series of 16 AYA patients with colon cancer, only 50 % fulfilled Amsterdam criteria, and 60 % of those not meeting Amsterdam criteria had a diagnosis of LS confirmed by molecular testing [11]. These results further support the general trend in recent years toward performing universal screening for LS on all incident colorectal cancer cases. Such screening involves relatively simple immunohistochemical analysis and/or microsatellite-instability testing (see below).
The mechanism underlying Lynch syndrome is defective DNA mismatch repair (MMR) genes. Germline mutations in the tumor suppressor genes MLH1 and MSH2 account for the majority of diagnoses; however, other genes including MSH6 and PMS2 have been implicated [12]. Furthermore, mutation of the EPCAM gene adjacent to MSH2 can result in changes to the promoter region and subsequent gene silencing of MSH2 [13]. DNA MMR defects result in accumulation of gene mutations, ultimately leading to tumorigenesis. The regions of DNA most susceptible to dysfunctional mismatch repair are areas known as micro- satellites, noncoding regions of repeating base pairs. These regions may increase or decrease in length, a finding known as microsatellite instability (MSI), that is, a hallmark of underlying deficiency of the MMR genes [9]. It is important to note, however, that the majority of tumors exhibiting MSI will not be due to Lynch syndrome. In fact, 10–15 % of sporadic colorectal tumors will also demonstrate MSI, but this is usually due to somatic MLH1 hypermethylation, leading to decreased protein transcription rather than germline gene mutations [9]. Irrespective of the etiology, tumors with MSI are associated with distinct prognostic and predictive characteristics that are discussed later in the chapter.
Recently, patients with homozygous or compound heterozygous (bi-allelic) MMR gene mutations have been reported. Such cases, referred to as constitutive MMR gene mutations, are often associated with a strikingly young age of cancer diagnosis. Most affected individuals will have evidence of café-au-lait spots, and CNS and hematologic malignancies are common. Many families have evidence of consanguinity, and in most instances, parents of affected children have no evidence of LS-related malignancies themselves [11, 14]. The spectrum on GI manifestations is extremely variable; however, when present, CRC is often diagnosed in adolescence, with a mean age of onset of 16 years in one series [15]. A heightened awareness of this syndrome is necessary when an AYA presents with malignancy, given the absence of relevant family history in most cases. In addition, many individuals present with non-colonic malignancies as their first presentation, with CRC as a subsequent malignancy. A set of surveillance strategies has been developed recently for constitutive MMR mutation carriers (Table 13.1).
Cancer type | Screening test |
|---|---|
Colon and rectum | Colonoscopy annually (beginning age 6) |
Upper gastrointestinal | Esophagogastroduodenoscopy annually (beginning age 6) |
Small bowel | Video capsule endoscopy annually |
Brain | Ultrasound at birth, MRI biannually |
Uterus | Ultrasound annually |
Urinary tract | Ultrasound annually |
13.3.1.2 Familial Adenomatous Polyposis
Several genetic syndromes predispose to polyp formation, which is often a precursor to invasive CRC. Polyposis syndromes include family adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP, an autosomal recessive disorder similar to FAP), characterized by adenomatous polyps. In contrast, Peutz-Jeghers syndrome, juvenile polyposis syndrome, and Cowden syndrome are often associated with hamartomatous and/or inflammatory polyps [7, 16, 17]. An exhaustive discussion of these syndromes is beyond the scope of this chapter, and, therefore, only FAP, the most common polyposis syndrome, will be discussed further.
FAP is an autosomal dominant condition occurring at a rate of 1/7,000 in the general population [18]. This syndrome classically manifests with hundreds to thousands of polyps throughout the colon. Around 95 % of affected patients will have polyps by age 35, but cases of polyposis with onset at ages as young as 5 years have been described [19]. Without surgical intervention, FAP carries at lifetime risk of invasive malignancy approaching 100 %, with median age of onset of 39 years. Invasive neoplasia affects 7 % by age 20 and 15 % by age 25. In addition to CRC, associated malignancies include duodenal adenocarcinoma and thyroid carcinoma [9].
FAP results from mutation of the adenomatous polyposis coli (APC) gene located on chromosome 5 that results in loss of function of this tumor suppressor gene [9, 20]. Mutations in different regions of the APC gene have been associated with variable phenotypes, and mutations of codon 1309 result in severe polyposis and a particularly young age of onset, with polyps sometimes developing in the first decade of life [21].
Patients with a family history of FAP should undergo colonoscopic screening for polyps starting at age 10–12 or 10 years earlier than the age at presentation of the youngest familial case [22]. Management ultimately involves prophylactic colectomy. Timing is individualized taking into consideration total adenoma burden, degree of dysplasia, mutational genotype, and emotional maturity of the patient. Three surgical approaches can be offered. Although most efficacious with respect to reducing cancer risk, total proctocolectomy with end ileostomy is least favored, due to requirement of a permanent ostomy. In most instances, subtotal colectomy and ileorectal anastomosis (IRA) or total proctocolectomy with ileal pouch-anal anastomosis (IPAA) is the treatment of choice. For IRA or IPAA, ongoing endoscopic surveillance is required due to cancer risk in the residual rectum or pouch [23].
13.3.2 Acquired Risk Factors
Beyond inherited syndromes, several predisposing factors for CRC in AYA have been described. Prior abdominal radiation, for example, in patients treated for childhood rhabdomyosarcoma, increases risk of CRC [24]. Screening with colonoscopy is recommended starting at age 35 or 10 years following radiation, whichever is later, for patients receiving ≥30 Gy of radiation to the pelvis [25]. Inflammatory bowel disease increases the risk of malignancy. Those diagnosed in childhood or adolescence carry a higher risk of malignancy in young adulthood [26]. For patients with ulcerative colitis, the cancer risk is 10 % for every decade of active disease beyond 10 years from diagnosis.
13.4 Biology
Compared to the very extensive study of CRC biology in older adults, there remains a paucity of data in AYA. However, there is a suggestion that the biology of CRC in this population demonstrates certain differences from that seen in older populations. Although some studies have suggested more right-sided tumors [27], others have in fact demonstrated a left-sided predominance [28]. Tumors often have a prominent mucinous component, are poorly differentiated, and display signet-ring cells. LaQuaglia et al. reported the presence of signet-ring cell histology in 45 % of cases in a cohort of 29 patients 21 years of age or younger with CRC [29]. Karnak et al. reported a prevalence of mucinous adenocarcinoma of 80 % in a series of 20 AYA patients with CRC [30]. In a review of pediatric CRC patients, mucinous features were present in 62 % of cases. In a larger study of 167 patients younger than 21 years of age, LaQuaglia confirmed a predominance of high-grade tumors with frequent signet-ring histology [27]. In SEER data comparing patients under 40 years of age with older patients, mucinous tumors constituted 21 % of the lesions in younger patients compared to 10–12 % in older adults, while the percentage of tumors that were poorly differentiated was 27 % compared with 15 % for older patients [31]. Similar histologic finding was reported in a large retrospective cohort study [28]. These histologic features are often associated with poor prognosis in older populations. However, such features are also common in tumors exhibiting MSI and in such instances do not appear to portent a poorer prognosis in older populations.
MSI is more common in AYAs with CRC compared to older patients, irrespective of whether it is related to underlying Lynch syndrome. Datta et al. reported MSI in 6 out of 13 patients who were under 21 years of age [32]. LaQuaglia et al. found MSI in 17 % of AYA patients [27]. However, unlike older populations, MSI does not appear to clearly correlate with distinct clinical, histological, or familial features compared to MSI-negative cancers in AYAs. Some studies in AYAs have suggested that, similar to MSI in older patients, MSI might be associated with better prognosis compared with microsatellite-stable tumors [27]. Microsatellite instability high (MSI-H) tumors also exhibit a significantly lower prevalence of k-RAS mutations that, when present, predict resistance to epidermal growth factor receptor (EGFR) inhibitors (see Management section 13.8.2.2).
Tumors without MSI in young adults appear to be associated with hypomethylation of proto-oncogenes, alluding to potential unique pathogenesis among this group of patients [33]. A study analyzing genomic complexity (gene copy number) and somatic mutation frequency in five genes critical to CRC development found that tumors from young adults tended to have more genomic complexity, P53 and PTEN mutations, and no PIK3CA mutations [34].
13.5 Presentation and Symptoms
The presenting symptoms of CRC in AYA, similar to older adults, are often nonspecific. With the exception of patients previously identified with a hereditary form of CRC predisposition, the diagnosis of CRC in asymptomatic AYA patients is uncommon. Vague, generalized abdominal pain is the most common symptom [29, 30, 35, 36]. Localizing abdominal pain is usually an indication of peritoneal involvement or perforation, indicating advanced disease locally. In this age group, localized pain is occasionally suggestive of appendicitis [37, 38]. Weight loss was noted in two thirds of young patients presenting with CRC in a review from St. Jude Children’s Research Hospital [24]. Other less frequent associated symptoms include nausea, vomiting, diarrhea, constipation, anorexia, rectal bleeding, pallor, abdominal distension, dysuria, and intestinal obstruction [29, 35, 36]. Symptoms generally relate to location of the primary tumor in the bowel. In young adults, the most common tumor location is the rectosigmoid followed by right-sided lesions. The former may cause changes in bowel habits and stool caliber, whereas the latter is more likely to present with symptoms associated with anemia. Very distal tumors may present with bleeding per rectum and tenesmus.
Studies suggest that there may be considerable delay in diagnosis of CRC in AYA, with time from symptom onset to diagnosis often exceeding 6 months [6, 29]. This is likely due to both patients and physicians having a low level of suspicion for a malignant diagnosis [2, 24]. Other contributors may include a sense of invincibility in AYAs and, in the United States, lack of medical insurance in this group [39, 40]. It has not been determined whether delay in diagnosis has a direct impact on outcome; however, it is apparent that CRC in AYAs is more likely to present at an advanced stage compared with older patients [30, 38]. In a retrospective review of pediatric CRC cases, 86 % had advanced disease at presentation, with more than 50 % of cases presenting with metastatic disease [27].
One study compared symptom burden among patients ages 18–39 to older adults (>40) diagnosed with CRC, most of whom where receiving chemotherapy and/or radiation. Young patients were more likely to report moderate to severe symptom scores for pain, fatigue, nausea, distress, dyspnea, drowsiness, and rash. This group reported that symptoms interfered with activity, mood, work, relations with others, and enjoyment of life to a greater degree than older patients [41].
13.6 Diagnosis and Staging
For diagnosis of CRC, colonoscopy is the investigation of choice, with the benefit of direct visualization of the mucosa and the ability to biopsy an abnormal lesion. It should be noted, however, that in the event of an obstructing malignant lesion, bowel preparation is unlikely to be tolerable, and in such instances patients may require definitive surgery without biopsy. Double-contrast barium enema offers low diagnostic yield and has largely been replaced by endoscopic investigation. Computed tomographic (CT) colonography is emerging as a useful test in screening for colorectal neoplasm; however it has a limited role in the workup of a symptomatic patient and therefore is rarely used in AYAs with suspected CRC [42].
For staging of CRC, CT scan of the chest, abdomen, and pelvis with lung and liver windows is the investigation of choice to identify distant metastasis. Both IV and oral contrast should be used for enhancement. Magnetic resonance imaging (MRI) of the pelvis is useful in staging of rectal tumors to assess for compromise of the mesorectal margin and/or regional lymph node enlargement [43, 44]. CT-PET may be useful in full staging of metastatic disease; however, currently this is not routinely recommended as a standard component of staging [45, 46]. Of note, positron emission tomography (PET) imaging is less sensitive at detecting metastasis in the setting of mucinous histology, a histologic feature more common in the AYA population compared to older patients. PET scan has been evaluated as a tool to identify the extent of metastatic disease prior to hepatectomy in the case of isolated liver metastasis. In a randomized trial, preoperative PET scan did not often change the surgical plan and hence is not recommended for routine use in this context [47].
Carcinoembryonic antigen (CEA) is a tumor marker commonly measured in older adults. When elevated, it may be a useful indicator of disease burden and response to treatment. This blood test has not been specifically studied in the AYA group.
Staging is performed following the American Joint Commission on Cancer tumor, node, and metastasis (TNM) guidelines [48]. This system is depicted in Table 13.2. Depth of tumor invasion, involvement of regional lymph nodes, and presence of metastases are combined to determine stage. Resulting subgroupings show significant differences in 5-year survival rates; hence stage is a powerful prognostic indicator.
Primary tumor (T) | Regional lymph nodes (N) | ||
|---|---|---|---|
TX | Primary tumor cannot be assessed | NX | Regional lymph nodes cannot be assessed |
T0 | No evidence of primary tumor | N0 | No regional lymph node metastases |
Tis | Carcinoma in situ | N1 | Metastases in 1–3 nodes |
T1 | Invades submucosa | N1a | Metastasis in 1 node |
T2 | Invades muscularis propria | N1b | Metastases in 2–3 nodes |
T3 | Invades through muscularis propria into pericolorectal tissues | N1c | Tumor deposit(s) in subserosa, mesentery, or nonperitonealized pericolic or perirectal tissues without regional nodal metastasis |
T4a | Tumor penetrates to the surface of the visceral peritoneum | N2 | Metastasis in four or more nodes |
T4b | Tumor directly invades or is adherent to other organs or structures | N2a | Metastasis in 4–6 nodes |
N2b | Metastasis in seven or more nodes | ||
Distant metastases (M) | |||
MX | Presence of distant metastases cannot be assessed | ||
M0 | No distant metastases | ||
M1 | Distant metastases present | ||
13.7 Prognosis
Five-year survival rates for CRC have been improving over time for both AYAs and older adult populations (see Fig. 13.3). Improvements have been less pronounced for AYA females compared to males. The results of some studies suggest poorer survival in the young [6, 40, 49], while other studies show comparable or better survival relative to patients diagnosed as having later-onset disease [50–52]. Between 2000 and 2011, 5-year survival rates were higher in AYAs compared to older adults with CRC. As an illustration, 5-year survival in stage 4 disease was 18.1 % in AYAs versus 6.2 % in the older group [50]. A very large cohort study recently reported outcomes of younger adults with CRC compared to older patients. Utilizing the US cancer registry database, greater than 13,000 patients 18–49 years of age were compared with 37,000 aged 65–75 years. Similar to previous findings, the younger cohort often presented with advantaged stage disease (62 % stages 3 and 4). However, stage-specific survival was similar for those presenting with stage 2 disease in the two groups, while survival was marginally better for younger adults with stage 3 and 4 disease. Importantly, the younger age group was statistically more likely to be treated with systemic therapy at all stages of disease presentation. This included a significant proportion of patients with stage 1 and low-risk stage 2 disease, in which chemotherapy is not considered standard of care [53]. Similar results were found in a cohort of patients with rectal cancer, in whom rates of radiation treatment were much higher in younger versus older patients, and stage-specific disease-free survival was similar in the two cohorts [54].
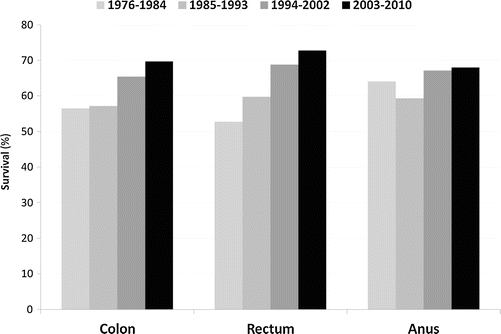

Fig. 13.3
5-year survival trends for invasive cancer of colon, rectum, and anus for patients aged 15–39, United States, SEER 1976–2010. Survival rates have improved over time (Ries L, Bleyer A, personal communication)
The issue of prognosis of CRC in AYA compared to older populations is therefore complex. On one hand, AYAs present at later stages of diagnosis, and the biology might be more aggressive. Conversely, AYAs have less comorbidity and are treated more aggressively than their older counterparts. It is not surprising, therefore, that data on prognosis in AYAs with CRC has been inconsistent thus far. Prospective data from registries of AYAs treated with modern chemotherapy, in the era of aggressive treatment of metastatic disease, will be required to further clarify prognosis.
13.8 Management of Colorectal Cancer
Treatment guidelines for young patients are usually extrapolated from adult trials, as the rare nature of CRC in AYAs precludes dedicated trials with adequate statistical power. A multidisciplinary approach is essential, and early referral to centers that are expert in the care of young patients with cancer will ensure the best possible outcome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree