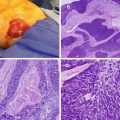
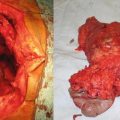
Fig. 16.1
Fearon and Vogelstein proposed genetic model of colorectal carcinogenesis. CRC is the result of accumulated mutations of multiple genes that are involved with cell growth and differentiation
Dietary Fat
Dietary fat and more specifically animal fat have been linked to a higher incidence of CRC [11, 14, 15]. The so-called western diet rich in animal fat has been incriminated due to a higher incidence of CRC in the western world. However, this concept has been challenged by well-conducted case control studies. An association with the total energy consumption rather than animal fat is thought to be responsible for a higher incidence of CRC [16].
Red Meat
The relationship between red meat consumption and an increased incidence of CRC is well established [17]. The proposed hypothesis is that red meat contains high amounts of heme, which in turn damages the colonic mucosa and stimulates epithelial proliferation. Also involved is the role of heme iron that is associated with the increase concentration of fecal N-nitroso compounds, a known carcinogen [18, 19].
Fruits and Vegetables
Due to their significant source of antioxidants, fruits and vegetables have been described in earlier studies as protective against CRC. However, more recent analyses provide conflicting conclusions on their protective/deleterious effect [20].
Dietary Fibers
Because of the low incidence of CRC in high fiber-consuming societies, it was a popular belief that high consumption of dietary fibers was protective against colon cancer. Burkitt has observed the clear differences in the incidence of CRC between the western world and areas of Africa. He concluded that high residue diet was protective by decreasing the transit time of stools and colon carcinogens. These same carcinogens were diluted by the bulk and size of the stools in a high fiber diet [21].
Some authors have challenged this. Studies have attempted to study the relationship between high consumption of fiber and low incidence of recurrent colorectal adenomas [22–26]. A meta-analysis concluded that there was no evidence to suggest that high dietary fiber will reduce the incidence or recurrence of adenomatous polyps within 2–4 years [27].
This being said, these studies were criticized for their weaknesses; compliance to a high fiber diet was not uniform in some of the patients, the duration of the studies was short, the arbitrary amount of daily fiber consumption designated as being sufficient was lower than the amount normally consumed by the high fiber-consuming societies, and patients had a lifelong exposure to a western diet before being enrolled in the studies. In a subsequent subgroup analysis, it was shown that it was possible to decrease the incidence of adenoma recurrence in the highly motivated and compliant patient, thus confirming the protective role of dietary fibers [28].
Insulin Resistance and Obesity
Obesity is linked to insulin resistance, increased levels of insulin, as well as increased activity of insulin-like growth factor type I (IGF-I). This hormone, in turn, is thought to be responsible for increasing cell proliferation, which can lead to an increased risk of developing colon cancer [29]. Weight gain in an adult is associated with an increase in colon cancer risk, thus stressing the importance of weight management as a measure for colon cancer prevention [30].
Folate and Alcohol
Folate is a vitamin commonly found in leafy vegetables, legumes, and some fruits. It plays a role in DNA methylation. A deficiency in folate may interfere with DNA repair and could be associated with certain cancers including CRC. Paradoxically, antifolate chemotherapy agents such as methotrexate and fluorouracil play an essential role in reducing the proliferation of neoplastic cell by inhibiting DNA synthesis. Supplementary folate could have either a beneficial or detrimental effect on CRC development, depending on the timing of the intake.
Recent data suggest that folic acid has a protective role in preventing CRC before its establishment. However, excess intake of folate will increase tumor genesis by providing nucleotide precursors to the multiplying neoplastic cells [31–33].
Alcohol has a role in decreasing the availability of folate in the body by altering its absorption. The consumption of alcohol has an association with CRC and seems to be related to the amount consumed. Compared to nondrinkers, moderate drinkers and heavy drinkers have a 21 % and 52 % increased risk of developing CRC, respectively [34].
Smoking
Bile Acids and Cholecystectomy
High fecal bile acid, mainly deoxycholic acid and lithocholic acid, appears to have a role in the increased incidence of CRC. Similarly, a cholecystectomy increases the quantities of fecal bile acid, resulting in an increase incidence of proximal colonic carcinoma [37].
Inflammatory Bowel Diseases
Crohn’s disease and ulcerative colitis (UC) are well-established risk factors for developing CRC. It seems that the duration of the disease, the extent of the colitis, and the severity of the inflammation are related to that increased risk. The age of onset of affected individuals at the time of diagnosis is generally 10–15 years younger than those with sporadic CRC [38].
Family History and Genetic Predisposition
There is a significant risk of developing CRC in individuals with a family history of CRC with a hazard ratio of 2.25 if a patient has a first-degree relative with CRC [39–41]. It is believed that the increased risk is attributed to genetic inheritance and/or exposures to similar environmental factors.
Inheritance of known susceptibility genes such as the APC gene, p53 gene, or the mismatch repair gene also predisposes an individual to developing CRC. Further discussion of the genetics associated with CRC is addressed in Chap. 20, Hereditary Colorectal Cancer and Polyposis Syndromes. It is important to stress that the majority of CRC associated with a family history have no known susceptibility genes.
Screening for Asymptomatic Colon Cancer
The goal of screening is early detection of a surgically resectable and curable cancer in an asymptomatic individual. Multiple tests are available to achieve that goal. For average-risk patients, screening should begin at age 50, whereas high-risk individuals should begin screening at age 40 or ten years prior to the diagnosis of the youngest affected family member.
The digital rectal examination (DRE) should be part of any routine examination in patients over 40 years of age, along with a fecal occult blood testing (FOBT) or a fecal immunochemical testing (FIT). The FOBT is an easy and inexpensive method to identify rectal masses and detect occult fecal blood. It is a proven method of decreasing the mortality from CRC. However, the test has inherent flaws, with approximately 50 % of patients with proven CRC having a negative result, while less than 10 % of test-positive patients will be found to have a CRC. Like all tests, FOBT has to be performed correctly by well-trained healthcare providers to optimize its value. Simply performing an office DRE with a fecal occult blood test or immunochemical testing is inadequate. The correct method is to collect three different stool samples at home. A special diet is recommended and should be free of any type of meat and high in fiber to stimulate bleeding and avoid false negatives. This test should be performed yearly as a screening method.
Colonoscopy is the only test that visualizes the entire colon and should be done every 10 years (Fig. 16.2). It is diagnostic because it can identify small polypoid lesions that would otherwise be missed by other screening modalities. Additionally, it is therapeutic because it can remove existing polyps. In addition to colonoscopy, FOBT should be performed yearly. Flexible sigmoidoscopy every 5 years is another test that can be used for screening. Double contrast barium enema every 5 years and, more recently, virtual CT colonoscopy every 5 years are offered as alternatives.
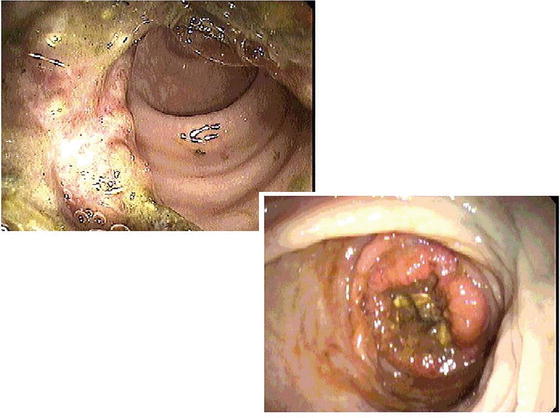

Fig. 16.2
Endoscopic view of colonic cancer (Courtesy of Michael Polcino, MD)
CT colonography (CTC) is also a screening option for average-risk individuals and has been endorsed by the American College of Radiology, the United States Multi-Society Task Force on Colorectal Cancer, and the American Cancer Society [8]. CTC has a staging accuracy of 81 %, with a sensitivity of 93 % and a specificity of 97 % for detecting polyps <1 cm. For polyps <1 cm, both sensitivity and specificity fall to 86 % and 86 %, respectively [42, 43]. The advantages with CT colonography include low risk of complications, minimal invasiveness, and high patient tolerance [44]. Although radiation exposure is a concern, the use of newer techniques has decreased the amount of radiation to a level that is close to background level. CT colonography does require colonic cleansing on the day prior to examination, and the patient is also instructed not to eat solids or dairy products a day prior to examination.
Fecal DNA testing is considered an evolving topic. Currently, it is not commercially available in the United States. The FDA molecular and clinical genetics panel of the medical devices advisory committee determined that Cologuard®, the first of its kind stool-based DNA screening test for CRC, has demonstrated safety, effectiveness, and a favorable risk benefit profile. This was based on a recent study that reported a sensitivity for detecting CRC of 92.3 % with DNA testing compared to 73.8 % with FIT (P = 0.002). The sensitivity for detecting advanced precancerous lesions (adenomas or large sessile serrated polyps) was 42.4 % with DNA testing and 23.8 % with FIT (P < 0.001) [45]. A positive test should be followed by a direct visualization of the colon by a colonoscopy.
Two categories of patients should be screened according to the American Cancer Society guidelines based on their risk factors (Table 16.1). The screening should begin by determining the individual’s level of risk, which could either be average risk or increased risk.
Table 16.1
Screening recommendations for colorectal cancer
Average risk (age > 50 years, without risk factors) |
Annual fecal occult blood test (FOBT) or † § |
Annual fecal immunochemical test (FIT) or † § |
Flexible sigmoidoscopy every 5 years or |
Colonoscopy every 10 years or |
Double contrast barium enema every 5 years or |
CT colonography every 5 years |
†Proceed with colonoscopy if test is positive |
§Take-home multiple sample should be used |
Increased risk |
Personal history of polyps removed |
Small rectal hyperplastic polyps: treat like average-risk individuals |
1–2 small tubular adenomas with low-grade dysplasia: colonoscopy 5–10 years after initial polypectomy |
3–10 adenomas or 1 adenoma > 1 cm or any adenoma with villous features or high-grade dysplasia: colonoscopy 3 years after initial polypectomy |
>10 adenomas on a single exam: colonoscopy < 3 years after initial polypectomy |
Sessile adenomas that are removed piecemeal: colonoscopy 2 to 6 months to confirm complete excision |
Personal history of CRC (had curative resection) |
Colonoscopy one-year anniversary after initial colon resection; if normal, then |
Repeat colonoscopy in 3 years; if normal, then |
Repeat colonoscopy in 5 years |
Following curative rectal cancer resection |
Periodic examination every 3–6 months for the first 2 to 3 years |
Family history |
First-degree relative (parent, sibling, or child) with CRC or adenomas diagnosed at age ≤60 years or two first-degree relatives at any age |
Colonoscopy every 5 years, beginning at age 40 years or 10 years before the age of the youngest affected relative (whichever comes first) |
First-degree relative with CRC or adenoma diagnosed at age >60 years, or two second-degree relatives with CRC |
Same options as average risk but begin at age 40 years |
High risk |
Inflammatory bowel disease |
Annual or every 2-year screening colonoscopy with biopsies for dysplasia. Significant risk of cancer begins 8 years after onset of pancolitis or 12 to 15 years after onset of left-sided colitis |
FAP or suspected to have FAP |
Annual screening sigmoidoscopy, beginning at age 10–12 years or refer for genetic testing |
HNPCC or suspected to have HNPCC |
Colonoscopy every 1–2 years, beginning at age 20–25 years; or 10 years younger than youngest age of CRC diagnosis in family, or refer for genetic testing |
For the average-risk patient, screening should start at age 50; the patient should be offered to choose from each screening method after explaining the advantages and disadvantages of each. The high-risk patient includes those with a personal history of adenomatous colon polyp or colon cancer, a family history of colon cancer, or a personal history of inflammatory bowel disease. A known family history of familial adenomatous polyposis (FAP) or hereditary nonpolyposis colon cancer (HNPCC) also puts the patient at an increased risk of developing CRC. These patients should be offered a screening colonoscopy at a younger age or at more frequent intervals.
An individual with a first-degree relative diagnosed with a CRC before age 60 should undergo a screening colonoscopy at age 40 or 10 years earlier than the affected family member, whichever comes first. A colonoscopy should then be offered every 5 years if no polyps are found.
An individual with proven FAP or at risk of FAP should have an annual sigmoidoscopy beginning at age 10–12. Genetic testing should be considered in these individuals. An individual with a genetic or clinical diagnosis of HNPCC should have a colonoscopy every 1–2 years starting at age 20–25 or 10 years earlier than the youngest age of colon cancer diagnosed in the family.
An individual with inflammatory bowel disease should have a surveillance colonoscopy with systematic random biopsies to rule out dysplasia. This should start 8–10 years after the onset of disease and be performed every 1–2 years.
The surveillance of individuals with a personal history of small hyperplastic polyps should be managed the same as those at average risk. However, those with hyperplastic polyposis syndrome are at an increased risk of developing adenomatous polyps and cancer and should therefore have more intensive follow-up according to the findings of the colonoscopy [46].
Presentation
Most CRC diagnosed are asymptomatic and are detected during a screening examination; moreover the majority of patients who develop CRC have no identifiable risk factor. For those who have symptoms, the location of the cancer usually dictates the symptoms. The most common presenting symptom is abdominal pain, and this can occur regardless of the location of the cancer or whether or not the tumor is obstructive. Change in bowel habits ranks second as the common complaint and varies from being a subtle change to a significant change. Change in frequency, shape, and consistency, usually presenting thinner or looser stools than usual, could be the alerting signs for CRC, especially if these symptoms persist. The location of the tumor can influence the timing of when these symptoms appear; compared to left-sided tumors, right-sided tumors tend to present with symptoms at a later time due to its larger colonic lumen, which can accommodate a larger tumor (Fig. 16.3).
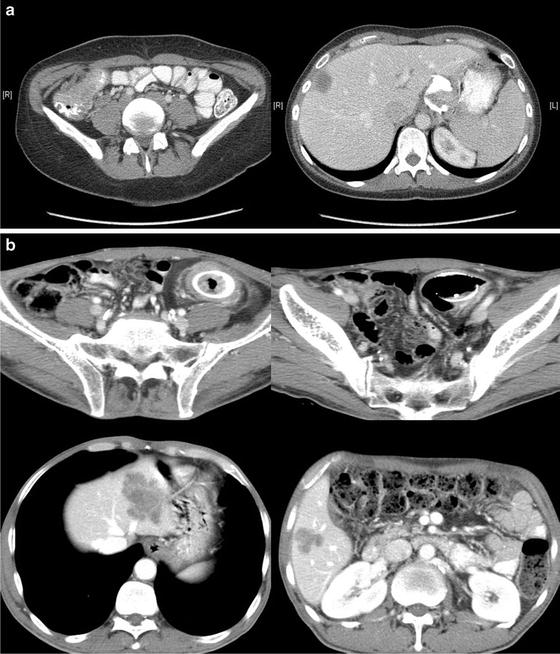

Fig. 16.3
(a) CT of a patient with a large cecal mass and metastasis to the liver. (b) CT of a patient with synchronous sigmoid mass and bilateral liver metastases. This patient had a colonic stent and underwent chemotherapy. He subsequently underwent a staged operation that included a sigmoidectomy, a left lateral segmentectomy and a right segmentectomy, rendering him disease free (A: Courtesy of Michael Polcino, MD) (B: Courtesy of Quyen D. Chu, MD, MBA, FACS)
Rectal bleeding could either be dark or bright red, depending on the location of the cancer, with bright red blood being associated with distal cancers. Very often this is a neglected symptom, and its presence should not be taken lightly. Patients with this complaint should be investigated, at the very least, with an endoscopy, even if they are young, to avoid a disastrous outcome stemming from the wrong assumption that such bleeding was due to symptomatic hemorrhoids. Hemorrhoids, if found, should be appropriately treated to avoid them from being blamed for persistent bleeding that may be attributed to cancer. Unexplained weight loss could be associated with advanced CRC, and when present, it usually portends a poor prognosis. Iron deficiency anemia is often due to a digestive disease and therefore deserves an investigation to avoid the risk of missing a malignancy [47].
In up to 15 % of the cases, a colon or rectal cancer will present as an obstructed or perforated cancer with septicemia, the latter of which can be difficult to distinguish from a perforated diverticulitis, thus making the management that much difficult [48].
Evaluation and Staging
When possible, every patient with a presumed or proven CRC diagnosis should undergo a full colonoscopy before initiation of treatment. Although the majority of patients will be diagnosed with a CRC after a full colonoscopy, some will be referred to a surgeon after an alternative method was performed (i.e., rigid sigmoidoscope). A tissue diagnosis must be obtained and synchronous carcinoma should be excluded, as the risk of synchronous carcinoma can be as high as 10 % in the general population [49–51]. In addition, synchronous benign polyps can occur in 13–62 % of cases [49–51], and when identified, they should be removed. In the event that a colonoscopy cannot be completed due to technical problems, a barium enema or a CT colonography and a rigid or flexible sigmoidoscopy should be performed.
Preoperative radiological staging is routinely performed. A CT of the chest abdomen and pelvis will help detect synchronous metastasis (Fig. 16.3); alternatively a PET CT of MRI could be obtained if an allergy to iodine is a concern.
A complete blood count and a carcinoembryonic (CEA) antigen level should be obtained. The latter test establishes a baseline value for which it can be used to compare to subsequent CEA levels during the surveillance phase to detect for possible recurrence.
Colon cancer is staged using the 7th edition of American Joint Committee on Cancer (AJCC)/TNM Staging system (Table 16.2) [53]. While distant disease can be detected prior to treatment, tumor depth (T-stage) and nodal involvement (N-stage) will only be known after surgical resection. The completeness of resection should be noted and designated by letter R where R0 represents a complete tumor resection, R1 represents an incomplete tumor resection because of involved microscopic surgical margin, and R2 represents an incomplete resection that leaves behind gross residual tumor. Histologic grade should also be noted as it plays a role in the prognosis and treatment consideration [54].
Table 16.2
American Joint Committee on Cancer (AJCC) TNM staging for colorectal carcinoma (7th edition)
Primary tumor (T)* | |
|---|---|
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ: intraepithelial or invasion of lamina propria* |
T1 | Tumor invades submucosa |
T2 | Tumor invades muscularis propria |
T3 | Tumor invades through muscularis propria into pericolorectal tissues |
T4a | Tumor penetrates to the surface of the visceral peritoneum** |
T4b | Tumor directly invades or is adherent to other organs or structures**, *** |
*Note: Tis includes cancer cells confined within the glandular basement membrane (intraepithelial) or mucosal lamina propria (intramucosal) with no extension through the muscularis mucosae into the submucosa | |
**Note: Direct invasion in T4 includes invasion of other organs or other segments of the colorectum as a result of direct extension through the serosa, as confirmed on microscopic examination (e.g., invasion of the sigmoid colon by a carcinoma of the cecum) or, for cancers in a retroperitoneal or subperitoneal location, direct invasion of other organs or structures by virtue of extension beyond the muscularis propria (i.e., respectively, a tumor on the posterior wall of the descending colon invading the left kidney or lateral abdominal wall, or a mid- or distal rectal cancer with invasion of prostate, seminal vesicles, cervix, or vagina) | |
***Note: Tumor that is adherent to other organs or structures, grossly, is classified cT4b. However, if no tumor is present in the adhesion, microscopically, the classification should be pT1-4a depending on the anatomical depth of wall invasion. The V and L classifications should be used to identify the presence or absence of vascular or lymphatic invasion, whereas the PN site-specific factor should be used for perineural invasion | |
Regional lymph nodes (N) | |
|---|---|
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Metastasis in 1–3 regional lymph nodes |
N1a | Metastasis in 1 regional lymph node |
N1b | Metastasis in 2–3 regional lymph nodes |
N1c | Tumor deposit(s) in the subserosa, mesentery, or non-peritonealized pericolic or perirectal tissues without regional nodal metastasis |
N2 | Metastasis in ≥4 regional lymph nodes |
N2a | Metastasis in 4–6 regional lymph nodes |
N2b | Metastasis in ≥7 regional lymph nodes |
Note: A satellite peritumoral nodule in the pericolorectal adipose tissue of a primary carcinoma without histologic evidence of residual lymph node in the nodule may represent discontinuous spread, venous invasion with extravascular spread (V 1/2), or a totally replaced lymph node (N1/2). Replaced nodes should be counted separately as positive nodes in the N category, whereas discontinuous spread or venous invasion should be classified and counted in the site-specific factor category tumor deposits (TD) | |
Distant metastasis (M) | |
|---|---|
M0 | No distant metastasis |
M1 | Distant metastasis |
M1a | Metastasis confined to one organ or site (e.g., liver, lung, ovary, nonregional node) |
M1b | Metastases > one organ/site or the peritoneum |
Anatomic stage/prognostic groups | |||||
|---|---|---|---|---|---|
Stage | T | N | M | Dukes* | MAC* |
0 | Tis | N0 | M0 | – | – |
I | T1 | N0 | M0 | A | A |
T2 | N0 | M0 | A | B1 | |
IIA | T3 | N0 | M0 | B | B2 |
IIB | T4a | N0 | M0 | B | B2 |
IIC | T4b | N0 | M0 | B | B3 |
IIIA | T1-2 | N1/N1c | M0 | C | C1 |
T1 | N2a | M0 | C | C1 | |
IIIB | T3-T4a | N1/N1c | M0 | C | C2 |
T2-T3 | N2a | M0 | C | C1/C2 | |
T1-T2 | N2b | M0 | C | C1 | |
IIIC | T4a | N2a | M0 | C | C2 |
T3-T4a | N2b | M0 | C | C2 | |
T4b | N1-N2 | M0 | C | C3 | |
IVA | Any T | Any N | M1a | – | – |
IVB | Any T | Any N | M1b | – | – |
Note: cTNM is the clinical classification, and pTNM is the pathologic classification. The y prefix is used for those cancers that are classified after neoadjuvant pretreatment (e.g., ypTNM). Patients who have a complete pathologic response are ypT0N0cM0 that may be similar to stage group 0 or I. The r prefix is to be used for those cancers that have recurred after a disease-free interval (rTNM) | |||||
*Dukes B is a composite of better (T3 N0 M0) and worse (T4 N0 M0) prognostic groups, as is Dukes C (any TN1 M0 and any T N2 M0). MAC is the modified Astler-Coller classification | |||||
Surgical Management
Surgical resection is the definitive treatment for colon cancer. The depth of bowel wall invasion and lymph node status are the two most important prognostic indicators. A definitive, oncologic resection for colon cancer should include a bowel resection which may require an en bloc resection of adherent structures and removal of the blood supply and lymphatics at the origin of the primary feeding vessel. For colon cancer surgery, 5 cm is an adequate proximal and distal margin so as to decrease the rate of anastomotic recurrence. This distance also allows for adequate nodal clearance. The 2000 National Cancer Institute guidelines for the surgical management of colon cancer recommend that a minimum of 12 lymph nodes should be examined [55]. Additionally, there is improved accuracy in the final pathologic state when there are more lymph nodes examined [56]. Tumor spillage should also be avoided to reduce risk of seeding the cancer into the peritoneum.
The surgeon should be cognizant of the status of the circumferential radial margin (CRM) (Fig. 16.4). CRM pertains to the non-peritonealized (part of the colon that is attached to the retroperitoneum) portion of the colon, and CRM is essentially the retroperitoneal margin. The non-peritonealized colon includes the cecum, ascending colon, descending colon, and upper rectum, areas that are fixed to the retroperitoneum and therefore are “immobile.” The fixed portion of the colon limits the surgeon’s ability to achieve a wider surgical margin. It is conceivable to appreciate how a bulky, posteriorly located cecal tumor is more likely to have a positive CRM than the same tumor whose bulk is located more anteriorly on the surface of the cecum. In contrast, mid-transverse colon and mid-sigmoid colon are mobile and are not likely to result in a positive CRM following resection.


Fig. 16.4
Circumferential radial margin (CRM) in colon cancer: The example is a cecal cancer. Note that a tumor that invades posteriorly into the retroperitoneal space runs the risk of having a positive CRM (panel A), whereas an anteriorly located tumor that penetrates into the serosa is not considered to have a positive CRM (panel B) (Redrawn by Quyen D. Chu based on data from Ref. [57])
A positive CRM is defined as tumor that is ≤1 mm from the non-peritoneal (i.e., retroperitoneal) surface of the specimen [58]. Although CRM for colon cancer may not be as well recognized as its rectal cancer counterpart, it nevertheless is important because it has prognostic significance. Patients with positive CRM are at risk of developing local recurrence [57, 59, 60]. Note that when the tumor encroaches on the serosal (peritoneal) surface but is not adherent to adjacent structures, it is not considered as having a positive surgical margin. This is an important distinction to make because a T3N0 tumor without CRM involvement does not normally require further treatment following resection, whereas the same tumor with a positive CRM may be treated with adjuvant therapy.
Because there is no anatomic landmark to differentiate the peritonealized and non-peritonealized portion of the colon, the surgeon should work closely with the pathologist to help indicate areas of the tumor that were in close contact with other organs and/or abdominal wall and specify the retroperitoneal margin. Full-thickness tumor with serosal involvement of the peritonealized colon could easily be confused as having a positive CRM when in reality, it represents a T3 lesion. Therefore it is imperative that the surgeon clarifies this subtle difference with the pathologist so as to ensure accurate recording on the final pathology report.
For colon cancer, both the open and minimally invasive techniques have been well described and studied. The discussion of the landmark studies comparing these techniques is done towards the end of this chapter. However, several technical considerations that are common to both surgical approaches shall be highlighted. Of note, safe colorectal oncologic surgery includes the clearance of lateral margins, resection of lymph node-bearing mesentery, and creation of a well-vascularized and tension-free anastomosis.
For a right colectomy, multiple approaches have been described with the two most common being the lateral-medial and the medial-lateral approach (Fig. 16.5). The important surgical tenets for an oncologic right colectomy are elevating the mesentery off of the retroperitoneum and duodenum, identifying and incising the lateral attachments, mobilizing the hepatic flexure, and taking care of not injuring the right ureter. The ileocolic artery, right colic artery, and right branch of the middle colic artery should be ligated at the origin in order to gain an optimal lymph node resection (Fig. 16.6). In the case of proximal transverse colon malignancies, an extended right hemicolectomy should be performed (Fig. 16.5). In the procedure, the entire middle colic artery is ligated at the origin. A well-vascularized and tension-free anastomosis between the ileum and transverse colon should then be created.
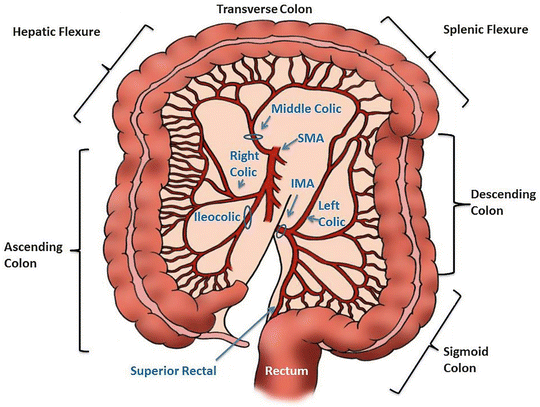
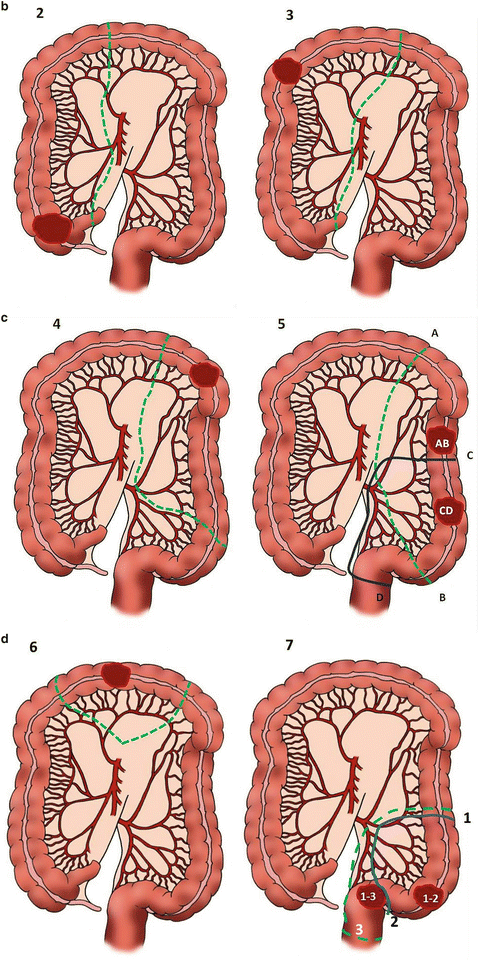
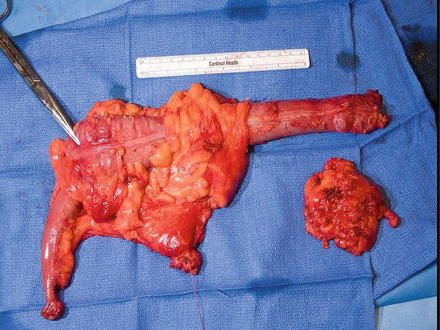


Fig. 16.5
Anatomy of the colon, its blood supply, and type of operation (A–D). Note that for cancer involving the cecum, ascending colon, hepatic flexure, and proximal transverse colon (Fig. 16.5B-2), the ileocolic artery, right colic artery, and the right branch of the middle colic artery are ligated. If the middle colic artery is ligated at its origin (Fig. 16.5B-3), then the distal bowel should be transected just to the distal third of the transverse colon to avoid bowel ischemia. Tumors in the splenic flexure (Fig. 16.5C-4) or descending colon (Fig. 16.5C-5) can be resected by taking the left colic artery without having to ligate the IMA at its origin. Alternatively, if the IMA is ligated at its origin, a sigmoidectomy will be required to ensure bowel viability. Tumors in the transverse colon can be approached with a segmental resection (Fig. 16.5D-6) or an extended right hemicolectomy (Fig. 16.5B-3) (Courtesy of Quyen D. Chu, MD, MBA, FACS)

Fig. 16.6
A right colectomy with ligation of its blood supply (Courtesy of Michael Polcino, MD)
For left colectomies, there are similar surgical principles as compared to the right colectomy (Fig. 16.5). The lateral attachments are incised, the mesentery is dissected free from the retroperitoneum, the left ureter is clearly identified, and the splenic flexure is mobilized. The mesentery is ligated at the origin of the main feeding vessel, either at the origin of the IMA or just distal to it at the left colic artery. The main feeding vessel ligated is contingent upon the exact location of the malignancy within the left colon. As mentioned previously, both the medial-lateral and the lateral-medial approach have been described. The medial-lateral approach begins with the identification of both the IMA and IMV. For a true splenic flexure lesion, the left colic artery can be ligated at its origin, thus preserving the remainder of IMA and its branches (Fig. 16.5). The splenic flexure can be approached from posterior as the mesentery is dissected free from the anterior surface of pancreas and Gerota’s fascia. Alternatively, the left colon can be dissected in the lateral to medial approach where the white line of Toldt is incised and the dissection is continued proximally until the left colon is completely free from the spleen and retroperitoneal attachments. The IMV is ligated as it enters below the inferior border of the pancreas in order to allow the mobilized proximal bowel to reach the pelvis. The origin of the IMA is ligated, which necessitates a sigmoidectomy to avoid anastomotic dehiscence from an ischemic sigmoid. Both approaches can be utilized as long as the vascular pedicle is ligated at the origin so as to ensure an adequate lymph node harvest.
In the past, it was thought that adhering to the “no touch isolation technique” by early ligation of the regional mesenteric vessels prior to mobilizing the primary tumor would reduce the occurrence of systemic disease. It was believed that such a technique would prevent cancer dissemination into the venous and lymphatic pathways. Unfortunately, this theory has been disproven. A randomized clinical trial of 237 colon cancer patients demonstrated no benefit with this technique [61]. Similarly, a study of over 1800 patients showed that early ligation of regional mesenteric vessels had no impact on the 5- and 10-year disease-free survival (DFS) as compared to late ligation after the tumor was mobilized [62].
Laparoscopic Versus Open Colectomy
Laparoscopic colon surgery has been practiced for the past 15–20 years. Over these past decades, at least 3 major randomized clinical trials and several systemic reviews and meta-analysis have demonstrated the oncologic equivalency of laparoscopic colectomy (LC) with open colectomy (OC) [63–66] (Table 16.3). The COST study (Clinical Outcomes of Surgical Therapy Study Group) was the first landmark study that demonstrated oncologic equivalency between LC and OC [63]. COST was a multicenter, prospective study that randomized 872 patients into two groups, LC and OC. The study demonstrated that not only was overall survival (OS) equivalent in both groups but also that there was no significant difference in cancer recurrence between them. At a median follow-up of 4.4 years, the 3-year OS rate was 86 % in the LC group versus 85 % in the OC group (P = 0.51), and the recurrence rate was 16 % in the LC group versus 18 % in the OC group (P = 0.32). Of note, the LC group had a shorter median hospital stay of one day (5 days vs. 6 days; P < 0.001) and briefer use of parenteral narcotics (3 days vs. 4 days; P < 0.001) and oral analgesics (1 day vs. 2 days; P = 0.02) compared to the OC group.
Table 16.3
Laparoscopic colon cancer trials
Trial, years | N | Median follow-up (mos) | Median # LNs harvested | DFS | OS | Comments |
|---|---|---|---|---|---|---|
COST [63], 2004 | 872 | 52.8 | LC: 12 OC: 12 | LC: 16 %a OC: 18 % (P = 0.32) | LC: 86 %a OC: 85 % (P = 0.51) | OR time was longer in LC group (150 min vs. 95 min; P < 0.001) |
LC had shorter hospital stay by 1 day, briefer use of narcotics by 1 day | ||||||
Complications were not significantly different between both groups | ||||||
794c | 62.9 | N/A | LC: 77 mosd OC: 89.5 mos (P = 0.589) | LC: 82.7 mosd OC: 78.3 mos (P = 0.78) | No significant differences in | |
Local recurrence rate | ||||||
Distant recurrence rate | ||||||
Wound/port-site recurrences | ||||||
COLOR [66], Buunen, 2009 | 1248 | 53 | LC: 10 OP: 10 (P = 0.32) | LC: 74.2 %a OC: 76.2 % (P = 0.70) | LC: 81.8 %a OC: 84.2 % (P = 0.45) | No significant differences in |
Morbidity and mortality | ||||||
Positive resection margin rate | ||||||
Local recurrence rate | ||||||
Distant recurrence rate |
Since the time of the COST study, there have been additional confirmatory trials. The CLASICC (Medical Research Council Conventional versus Laparoscopic-Assisted Surgery in Colorectal Cancer) [64] and the COLOR (Colon Cancer Laparoscopic or Open Resection) Study Group are the two additional prospective randomized studies that produced similar results as the COST study [64, 66].
The CLASICC trial randomized 794 patients with both colon and rectal cancer to either LC or OC. There was no significant difference in the DFS or OS between the two groups. The 3-year DFS was 66.3 % for the LC versus 67.7 % for the OC group (P = 0.70), and the 3-year OS was 68.4 % for the LC group versus 66.7 % for the OC group (P = 0.55) [64]. In their long-term follow-up of more than 10 years, they reported the durability of their initial findings. The DFS was 77 months for the LC group versus 89.5 months for the OC group, and the OS was 82.7 months for the LC group versus 78.3 months for the OC group (P = 0.78) [65]. There were no differences in distant recurrences between OC and LC groups; the 10-year rates were 19.8 % and 22.7 %, respectively (P = 0.588). Finally, the incidence of wound/port-site recurrences was also not significantly different.
The COLOR trial randomized 1248 patients to either LC or OC. At a median follow-up of 53 months, there was no significant difference in DFS or OS between the two groups. The 3-year DFS for the LC and OC was 74.2 % and 76.2 %, respectively (P = 0.70), and the 3-year OS for the LC and OC was 81.8 % and 84.2 %, respectively (P = 0.45). There were no significant differences in the morbidity and mortality rates, positive resection margin rate, local recurrence rate, or distant recurrence rate between LC and OC [66].
Robotic surgery has recently emerged as an alternative minimally invasive technique to laparoscopic colon surgery. The benefit of robotic colon cancer surgery as compared to laparoscopic surgery is currently being evaluated. Specific attention is currently being directed towards intracorporeal anastomoses for right colon cancer surgery [68].
In summary, laparoscopic colectomy yielded similar oncologic outcomes as open colectomy; OS, DFS, local recurrence rate (i.e., port-site recurrence), distant recurrence rate, and long-term quality of life were not significantly different between the LC group and the OC group [65]. Unlike laparoscopic proctectomy for rectal cancer (please see Chap. 18 , Rectal Cancer), laparoscopic colectomy has been accepted by the healthcare community as a viable option for patients with colon cancer.
Systemic Therapy: Overview
Stage I colon cancer has an excellent prognosis with a 95 % 5-year survival after resection and therefore does not require adjuvant systemic therapy. Stage II disease also has a favorable prognosis with a 5-year survival rate in the range of 70–80 % after surgical resection. The role of adjuvant systemic therapy is controversial. Stage III disease, on the other hand, has a 40–60 % survival after curative resection. Adjuvant systemic chemotherapy for stage III colon cancer is standard of care. Adjuvant therapy for stage II colon cancer is controversial and will be discussed after the discussion of adjuvant therapy for stage III colon cancer, which is well established.
Adjuvant Therapy for Stage 3 Colon Cancer
The 5-year survival for patients with resected stage 3 colon cancer (positive lymph nodes, irrespective of T-status) is in the range of 75 %. Adjuvant systemic therapy significantly improves this rate to nearly 79 %. In 1990, the Intergroup Trial INT-0035 was the first to report a significant reduction (33 %) in the risk of death with one year of adjuvant 5-FU and levamisole in patients with stage 3 colon cancer [69]. These findings were later confirmed in 1995 [70, 71]. Subsequently, one year of levamisole was replaced with 6 months of leucovorin such that up until 2004, the current standard of care for patients with stage 3 colon cancer was 6 months of 5-FU and leucovorin (5-FU/LV) [72–74]. More recent data established the efficacy of adding oxaliplatin to the fluoropyrimidine-based chemotherapy [75–77] (Table 16.4).
Table 16.4




Clinical trials evaluating the role of adding oxaliplatin to fluoropyrimidine-based chemotherapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree