Study
Patients
Chemotherapy type
Time of assessment
Age
Cognitive assessment
Results
Mayerhofer et al. [27]
Patients with advanced ovarian cancer (n = 28)
Paclitaxel/carboplatin
Before CT, after cycle 3, and after CT completion
Median: 63 (54–69)
NP tests: attention, motor skills
82 % of patients had impairment before CT (mainly motor skills)
No decline during or after CT
Improvement in attention scores
Hensley et al. [20]
Patients with advanced ovarian, peritoneal, or fallopian tube cancer (n = 20)
Paclitaxel, carboplatin, gemcitabine
Before CT, after cycles 3 and 6, and 6 months after CT completion
Median: 54 (25–70)
2 NP tests: executive functions, processing speed, short-term and working memories
Subjective assessment
No decline during or after CT
Hess et al. [28]
Patients with advanced ovarian or primary peritoneal cancer (n = 27)
Platinum-based therapy
Before CT, after cycles 3 and 6
Mean 59 (40–82)
NP computerized tests: attention, processing speed, reaction time
Subjective assessment
92 % decline at cycle 3 and 86 % decline at cycle 6 (compared to baseline)
About 40 % of patients had ≥2 domains impaired
Correa et al. [29]
Ovarian cancer survivors in complete remission (n = 22, group 1) vs. ovarian cancer survivors with recurrent disease receiving CT (n = 26, group 2)
Mainly paclitaxel and carboplatin
Cross-sectional: 5–10 years from diagnosis
Group 1: 62 ± 9.2
Group 2: 60 ± 9.1
Battery of standardized NP tests: attention, executive functions, learning and memory abilities
No difference between patient groups
28 % of patients had cognitive impairment
Mayerhofer et al. [27] assessed cognitive functioning before treatment, after 3 cycles, and at the end of chemotherapy (paclitaxel/carboplatin) in 28 women (median age 63) with advanced ovarian cancer. Attention and motor skills were assessed with neuropsychological tests. Before starting chemotherapy, 82 % of patients presented cognitive impairment (mainly motor skills), and high rates of anxiety were observed. During and after treatment, patients with initial deficits did not deteriorate. On the other hand, a significant improvement on attention scores was found (p < 0.05) probably owing to practice effects. Thus, the results showed no signs of neuropsychological worsening after paclitaxel/carboplatin chemotherapy.
Hensley et al. [20] studied the impact of chemotherapy (paclitaxel, gemcitabine, and carboplatin) on cognitive functioning and quality of life in 20 women with advanced ovarian, peritoneal, or fallopian tube cancer (median age 54). Four evaluations were performed: before treatment, after cycles 3 and 6, and 6 months after completion of chemotherapy. Patients had a short objective (2 tests of executive functions and processing speed) and subjective assessment with a self-report questionnaire investigating memory and concentration. Results showed that objective cognitive functioning did not decline during or after chemotherapy. More highly educated women (i.e., more than 16 years of education) reported a decline in their concentration, memory, and emotional well-being during treatment. Cognitive complaints returned to baseline levels 6 months after completion of chemotherapy. Moreover, depression and overall quality of life scores did not change significantly during or at completion of chemotherapy. However, cognitive assessment was insufficiently detailed in this study so it is difficult to draw any conclusions.
Hess et al. [28] investigated objective and subjective cognitive changes among women with newly diagnosed advanced ovarian cancer (n = 27, mean age = 59) with assessments prior to chemotherapy and at the third and sixth cycle of chemotherapy (platinum-based therapy). Objective assessment consisted in web-based evaluation of attention, processing speed, and reaction time. Results showed that 92 % and 86 % of patients demonstrated cognitive declines at least on one domain from baseline to cycle 3 and from baseline to cycle 6, respectively. Forty-eight percent and 41 % of patients had at least two domains impaired at cycle 3 and cycle 6, respectively. Nevertheless, higher levels of memory complaints were reported before than after chemotherapy.
One study focused on the cognitive functioning of long-term survivors of ovarian cancer. The cross-sectional study of Correa et al. [29] in women diagnosed with ovarian cancer 5–10 years prior to study enrollment compared neuropsychological performances of women in complete remission (n = 22) with that of women who had recurrent disease and were receiving chemotherapy (n = 26). Patients in both groups received chemotherapy prior to enrollment (mainly paclitaxel and carboplatin). Twenty-eight percent of patients had cognitive impairment, which is greater than would be expected considering healthy population norms (p = 0.03). In the group of survivors who were treated for a disease relapse, there was a trend for a higher frequency of impairment than that reported in healthy population norms (p = 0.051), whereas this result was not found in women in complete remission. In the group of recurrent disease, there was a moderate negative correlation between the number of prior chemotherapies and performances on tests of attention and executive functions. Furthermore, no significant difference was found between these two groups of patients on tests of attention, learning and memory abilities, and executive functions or depression.
Cognitive complaints have also been reported in studies using the self-report questionnaire QLQ-C30 of the European Organization for Research and Treatment Cancer (EORTC) in ovarian cancer patients [22]. With this questionnaire, a decline in perceived cognitive function was observed in some but not all studies [22]. However, the QLQ-C30 includes only 2 items dedicated to cognition and it was not designed and developed specifically to assess cognitive complaints.
In summary, longitudinal studies are not consensual regarding the deleterious impact of chemotherapy on cognitive functioning in ovarian cancer patients. These inconsistent findings are likely to be in part related to methodical issues [22]. The studies included small sample sizes and no control group to help to delineate the effect of chemotherapy on cognition (cancer patients not treated by chemotherapy and healthy controls to apprehend practice effects), and only a few neuropsychological tests were used and not all the domains likely to be affected by chemotherapy were assessed. Furthermore, cognitive complaints were not assessed with a specific questionnaire like the Functional Assessment of Cancer Therapy–Cognitive Function (FACT-Cog), which has been validated with cancer patients [30]. Moreover, the longitudinal studies focused only on the acute effect of chemotherapy, and the impact of antiangiogenic agents such as bevacizumab in association with chemotherapy and in maintenance was not assessed.
The four studies presented were not dedicated to elderly patients and only a few older women with ovarian cancer were included.
Additional research with a longitudinal design and following the International Cognition and Cancer Task Force recommendations [31] is therefore needed to apprehend more precisely the contribution of the disease, treatments, and other risk factors on cognitive functioning linked to aging.
Elderly Cancer Patients
The chemobrain has been particularly studied among women treated for breast cancer, mainly in middle-aged patients (40–50 years). While chemotherapy is more commonly proposed to elderly cancer patients, little is known about its impact on cognitive functioning. Among the published studies that have assessed the impact of ovarian cancer and its treatments on cognitive functioning, none focused on elderly patients.
Specificities of Elderly Cancer Patients
Aging is associated with cognitive and functional decline and comorbidities that may have a significant impact on the patient’s autonomy. These alterations may be exacerbated by cancer and the toxicity of antitumoral agents, and functional decline is associated with a worse prognosis. Initial cognitive functioning and functional status could thus influence the choice of treatment.
The impact of cancer and chemotherapy on cognition and quality of life is thought to depend on several factors including baseline cognitive functioning, which is expected to be lower in older patients than in younger ones [32]. Little is known about the effect of treatment on the cognitive functioning of older patients [33] or whether chemotherapy-associated cognitive alterations affect an older patient’s ability to perform daily activities.
Studies in Elderly Cancer Patients
Although there is no data available in ovarian cancer patients, the first longitudinal study on cognitive assessment in older patients with neuropsychological tests was conducted among breast cancer patients treated by adjuvant chemotherapy (n = 28, mean age 71 ± 5) [33]. Thirty-nine percent of patients experienced a decline in cognitive performance within the 6 months following the start of chemotherapy (50 % had no change and 11 % improved). Furthermore, cancer survivors were also concerned by cognitive impairments: breast cancer survivors, who remained disease-free for more than one or two decades (mean age 73 ± 5.1 and 64 ± 6.4, respectively), performed worse on average than population controls [13, 34]. A similar pattern of differences in neuropsychological performances was found between groups to the detriment of the patient groups having undergone long-term chemotherapy. The impairment of executive functioning and psychomotor speed, working memory and attention, immediate and delayed verbal memory, and information processing speed [13] was in favor of dysfunctions involving the fronto-subcortical brain regions. Overall, the low number of studies performed in elderly patients suggests that cognitive disturbances affect the same functions as in younger patients. Previous cancer treatment may therefore exacerbate cognitive dysfunctions associated with age-related brain changes.
On the other hand, studies using cognitive screening tests like the Mini Mental State Examination or MMSE [35] did not find any cognitive impairment after cancer treatment. Cognitive impairments induced by cancer treatment are subtle and screening tests did not appear sensitive enough to detect them. However, even a small decline in elderly patients may have an important impact on their daily life.
Aging, Cognitive Decline, and Cancer
Many mechanisms are involved in cognitive deficits particularly in elderly cancer patients (Fig. 2.1 [36]). Aging, neurodegeneration, biological processes underlying cancer, the impact of cancer treatments, and cognitive decline appear to be linked, leading to the phase shift hypothesis, i.e., that cancer treatments may accelerate the aging process. According to this hypothesis, age-associated decline in cancer patients is not only parallel but is greater than that of older adults with no cancer history. An additional hypothesis, which does not exclude the latter, postulates that only vulnerable populations exhibit the accelerated aging pattern [5].
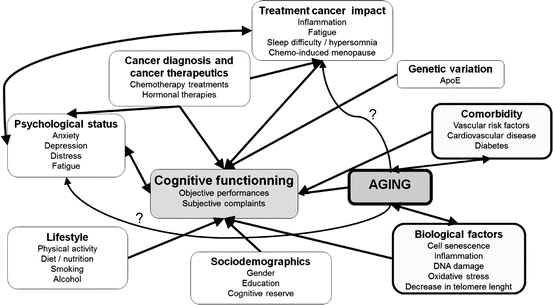

Fig. 2.1
Possible contributors to cognitive difficulties in elderly cancer patients (issued from Lange et al. [32], Cancer Treat Rev)
Some factors closely related to aging such as comorbidities (mainly cardiovascular disease, vascular risk factors, and diabetes), cognitive frailty, biological factors, tolerance to cancer treatment, and psychological status may contribute to exacerbating cognitive difficulties. Aging may potentially impact inflammation, oxidative stress, cell senescence, and DNA damage and lead to a decrease in telomere length, which may cause cognitive deficits [17, 37–40]. Physiological factors such as the patient’s genetic makeup (e.g., the Apolipoprotein E (ApoE) associated with cognitive impairment related to Alzheimer’s disease, brain trauma, and aging [41]), hormone levels, the inflammatory response to cancer treatments, postoperative cognitive dysfunctions, fatigue, and sleep difficulty or hypersomnia could also affect cognition. The patient’s psychological status including degree of anxiety, depression, and stress increases vulnerability regarding cognitive function [42]. Lastly, it may be that other risk factors, such as low socioeconomic status, diet and nutrition, sedentary lifestyle, or alcohol and tobacco consumption, influence the development of both cancer and cognitive impairment [43]. However, the main cause of neuropsychological deficits in patients treated for cancer is chemotherapy [44].
Perspectives to Assess and Improve Cognition in Elderly Ovarian Cancer Patients
More than half of patients with ovarian cancer are over 65 at diagnosis [2]. Nevertheless, age is a risk factor for ovarian cancer and cognitive impairment. Unfortunately, the few studies assessing the impact of cancer and treatment among ovarian patients include very few patients over 65. Furthermore, these cancer patients usually had a general anesthesia of long duration, which could induce postoperative cognitive dysfunction, especially in elderly patients [45]. Moreover, the management of ovarian cancer includes multiple courses of chemotherapy, and a meta-analysis has shown that treatment of longer duration (i.e., the number of cycles of chemotherapy received) can lead to poorer cognitive performance [46].
While it is well established that chemotherapy induces cognitive impairment, there is emerging evidence suggesting that maintenance therapy with antiangiogenic agents plays a role in brain cognition and may induce neurotoxic effects on cognitive function [4, 47]. Some comorbidities, like hypertension, should be well controlled to limit the risk of cerebral toxicity.
Recommendations
Studies in elderly patients require a longitudinal design to assess cognition before any treatment insofar as these patients are more likely to exhibit age-related cognitive impairment before treatment compared to younger patients. Thus, future research should take into account the impact of chemotherapy and antiangiogenic treatment on cognition in order to better understand the trajectory of cognitive decline. Sufficient numbers of older patients and geriatric assessment of functional status should be included. Indeed, therapy-associated cognitive changes may affect an older patient’s ability to perform daily activities. Cognitive assessment should also include batteries of neuropsychological tests rather than screening tests and should target cognitive domains hypothesized to be impacted by treatment, such as memory, executive functions, attention, and information processing speed. Such assessment should not be too long owing to the potential impact of fatigue in elderly patients [33].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree