Cognitive Decline Following Cancer Treatment
James C. Root
Elizabeth Ryan
Timothy Ahles
INTRODUCTION
Cognitive decline following cancer diagnosis and treatment is an important clinical problem for cancer survivors that may have far-reaching consequences in home-life, educational attainment, and vocational success. Increasingly, cognitive decline has been identified in individuals treated for non-CNS cancers, in which no primary neurological etiology can be identified. Reports from patients and their physicians of cognitive decline in breast and other cancers following treatment has led to a focus on various treatment modalities and their potential effect on cognition in these groups. While earlier reports generally focused on the effects of chemotherapy exposure (“chemobrain”), other factors and treatment modalities are increasingly recognized as potential contributors to ultimate cognitive dysfunction. In addition to chemotherapy exposure, hormone treatment, pain medications, genetic susceptibilities, immune system dysfunction, and the psychological and physical stress of diagnosis and treatment may all potentially affect self-report and objective findings of cognitive dysfunction. For this reason, we focus on post-treatment difficulties in cognition with a focus on chemotherapy exposure, mainly derived from studies in women with breast cancer, but with the caveat that several other factors may also contribute to reported and observed dysfunction. Studies utilizing neuropsychological measures have defined the objective cognitive effects following treatment and have been extended through the use of structural and functional neuroimaging to better understand potential pathology underlying cognitive dysfunction. In addition, while still in the early stages of investigation, mechanisms by which cancer treatments might influence cognitive abilities and brain function have been proposed that might help to explain resulting cognitive dysfunction.
In this chapter, we will first describe a typical case of cognitive dysfunction putatively associated with cancer treatment to provide the reader a better understanding of how this syndrome presents in a routine clinical setting. We will then review research literature documenting the self-report of individuals’ post-treatment in regard to their most prominent cognitive symptoms and how these affect day-to-day functioning. Research documenting formal, objective measurement of cognitive abilities in this group is then reviewed and contrasted. Contributions of structural and functional imaging, which may help to clarify the underlying changes in brain structure and function following treatment, are then discussed, followed by potential mechanisms by which treatment may exert an effect on the brain and cognition. We then close with a review of the emerging literature on cognitive rehabilitation and pharmacologic interventions that have been developed for the treatment of cognitive changes following treatment.
CASE SUMMARY
Ms. C., a 25-year-old woman diagnosed with non-Hodgkins lymphoma approximately 2 years before, was referred to our service with reports of forgetfulness, poor concentration, and difficulties in understanding instructions and learning new tasks. Ms. C. had undergone chemotherapy treatment consisting of four rounds: rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone (R-CHOP), and two rounds: ifosfamide, carboplatin, and etoposide (ICE) after her diagnosis, with no evidence of disease since then. While she had had no difficulties performing her work in office administration before treatment, she found herself making increasing errors on routine assignments, felt more easily distracted, and experienced difficulties when new tasks were assigned to her. These difficulties only increased when she took a new position with similar responsibilities in a different, faster-paced environment with new co-workers. She found herself making significant errors on a daily basis and increasingly relying on her co-workers around her for additional help, both of which led to reprimands from her supervisors. Ms. C. expressed considerable anxiety about her situation, fearing that her many mistakes were putting her job in jeopardy.
Ms. C. was administered a standard neuropsychological test battery, which included measures of processing speed, attention, language functioning, verbal and visuospatial reasoning, learning and memory, as well as executive functioning; psychological and emotional factors that may have been contributing to the reported issues were also assessed by self-report. Our assessment found mildly impaired cognitive and motor slowing across all timed tasks in which speed and efficiency are prerequisites, as well as difficulties in recollection of previously learned information. Complicating the clinical picture, Ms. C.’s profile was also notable for considerable anxiety, manifested by rumination, tension, and constant worry. These difficulties were discussed with Ms. C., who found the objective results broadly overlapping with the issues she had identified at work and at home; specifically, she identified feelings of being forgetful, “slower,” “less efficient,” difficulties in real-time, pressured situations, and
constant anxiety that appeared in reaction to mistakes as well as distracted her from tasks at hand. Cognitive rehabilitation, tailored to her specific tasks and difficulties at work, was recommended, as well as regular psychotherapy for treatment of the attendant anxiety in relation to her diagnosis and current difficulties.
constant anxiety that appeared in reaction to mistakes as well as distracted her from tasks at hand. Cognitive rehabilitation, tailored to her specific tasks and difficulties at work, was recommended, as well as regular psychotherapy for treatment of the attendant anxiety in relation to her diagnosis and current difficulties.
SELF-REPORTED COGNITIVE DECLINE FOLLOWING TREATMENT
The kinds of difficulties described above are widely cited in literature studying self-reported cognitive issues following treatment. Slowing, inattention, distraction, forgetfulness, difficulties in multi-tasking, and language function are all implicated in patients’ self-report to varying degrees. Early research on self-reported cognitive difficulties in mixed etiology cancer patients found that roughly half of patients reported difficulties in memory or concentration at some point in their treatment (1). Follow-up by this group in a sample of 91 lymphoma patients six or more months posttreatment found 30% of patients reported difficulties in concentration, with significant forgetfulness being reported by 52% of patients (2). That these self-reported cognitive difficulties persisted for longer interims post-treatment was confirmed by Schagen et al. (3), who found persistent reported cognitive difficulties, including poor concentration (31%) and forgetfulness (21%) in breast cancer survivors treated with cyclophosphamide, methotrexate, and fluorouracil 5FU (CMF) compared with surgery and radiation treatment alone. Similarly, Ahles et al. (4) found persistent reports of difficulties in concentration and complex attention up to 10 years post-treatment in individuals who received systemic chemotherapy for breast cancer and lymphoma. Several other studies, including patients with different cancers at various time points during and after completion of treatment, have found similar increased incidence of reported cognitive difficulties putatively associated with chemotherapy and hormone exposure (5,6,7,8,9,10,11,12,13).
OBJECTIVE MEASUREMENT OF COGNITION FOLLOWING TREATMENT
The first studies that investigated objective differences in cognition of individuals post-treatment were cross-sectional, contrasting individuals post-treatment with healthy controls or cancer diagnosed controls undergoing a different treatment. Initial cross-sectional studies of breast cancer survivors found that 17% to 75% of patients experienced cognitive difficulties anywhere between 6 months and 10 years post-treatment. A meta-analysis that included mainly cross-sectional studies (5,6, 14) found small to moderate effect sizes across motor function, executive function, learning and memory, spatial reasoning, and language function; these results square with reports of cancer survivors and their clinicians, which document difficulties including forgetfulness, difficulties in attention and multi-tasking, cognitive slowing, and difficulties in word finding (15,16). Ahles et al. (4) documented that a subset of cognitive difficulties appear even at longer intervals post-treatment, with chemotherapy-exposed individuals exhibiting significantly worse performance overall and lower performance specifically in verbal ability and psychomotor speed 10 years post-treatment.
The cross-sectional design of these studies, which do not include pre-treatment baseline assessments, qualifies the interpretation of their results. Cancer patients may present with pre-treatment, baseline cognitive dysfunction, with these difficulties persisting through treatment and posttreatment testing (17,18). Wefel et al. (18) found that 35% of women pre-chemotherapy treatment exhibited cognitive impairment. Ahles et al. (2008) investigated pre-treatment cognitive ability in healthy controls and patients diagnosed with invasive (stages I to III) and non-invasive (stage 0) breast cancer and found significantly slowed reaction time in the invasive group compared with healthy controls, and lower overall performance in the invasive group compared with the healthy and non-invasive patient groups. While pretreatment/baseline differences remain poorly understood in regard to mechanism or etiology, the fact that differences are present between groups prior to treatment requires that longitudinal assessments be conducted.
More recent longitudinal studies that can control for these pre-treatment differences have contrasted cancer diagnosed individuals undergoing different treatment regimens with each other and with healthy, non-cancer diagnosed individuals. Results of these longitudinal studies suggest more specific and more subtle cognitive decline associated with chemotherapy treatment than previous cross-sectional studies would indicate. A subset of these studies find no observable cognitive effect of chemotherapy exposure (19), observable decline in only a subset of patients treated with a specific regimen of chemotherapy (CTC vs. FEC) (20), or declines in only a subset of cognitive abilities with no overall decline in neuropsychological performance (21). Positive studies generally suggest that declines in performance following chemotherapy exposure will be in specific cognitive abilities and that these declines will only be exhibited in a subset of individuals. Quesnel et al. (22) compared chemotherapyexposed and radiotherapy-treated patients pre-treatment and at 3 months post-treatment and found that verbal memory was affected in both groups regardless of treatment, but with a specific effect on verbal fluency in the chemotherapyexposed group.
Recent work complicates the interpretation that chemotherapy, in isolation, is responsible for cognitive decline post-treatment and reinforces the argument that effects posttreatment are likely multi-factorial. In addition to chemotherapy treatment, longitudinal studies that included patients not exposed to chemotherapy and healthy controls revealed that the no chemotherapy group frequently performed as poorly as the chemotherapy group or at an intermediate level between the chemotherapy-exposed group and the healthy controls. This pattern of results raised the question of whether endocrine therapy could impact cognitive functioning. Initial examination of this issue produced mixed results; however, most
studies were not powered to adequately examine the independent effects of endocrine therapy. A recent longitudinal study examining patients not treated with chemotherapy who were randomized to tamoxifen or exemestane revealed that patients treated with tamoxifen, but not with exemestane, experienced cognitive problems compared with healthy controls (23). Even though investigators assumed that they were studying the effects of chemotherapy, in reality, most breast cancer patients receive multi-modality treatment including surgery with exposure to general anesthesia, radiation therapy, and endocrine therapy in addition to chemotherapy. This, in combination with the evidence for pre-treatment cognitive issues, lead Hurria and colleagues to propose the phrase “cancer and cancer treatment-associated cognitive change” as a more accurate descriptor of the phenomenon (24).
studies were not powered to adequately examine the independent effects of endocrine therapy. A recent longitudinal study examining patients not treated with chemotherapy who were randomized to tamoxifen or exemestane revealed that patients treated with tamoxifen, but not with exemestane, experienced cognitive problems compared with healthy controls (23). Even though investigators assumed that they were studying the effects of chemotherapy, in reality, most breast cancer patients receive multi-modality treatment including surgery with exposure to general anesthesia, radiation therapy, and endocrine therapy in addition to chemotherapy. This, in combination with the evidence for pre-treatment cognitive issues, lead Hurria and colleagues to propose the phrase “cancer and cancer treatment-associated cognitive change” as a more accurate descriptor of the phenomenon (24).
The research cited above confirms the presence of objective cognitive difficulties in a subset of cancer patients posttreatment, and these difficulties have been linked specifically to chemotherapy treatment as well as to several other variables, including endocrine therapies. Significantly, while both subjective reports and objective assessment have found significant changes post-treatment, outcomes appear to differ depending on whether cognition is assessed by self-report or neuropsychological testing.
SUBJECTIVE VERSUS OBJECTIVE MEASUREMENT OF COGNITION FOLLOWING TREATMENT
Similar to findings in other disorders, subjective cognitive reports exhibit poor agreement with results of objective testing at the level of the individual. Depending on the study, this discrepancy appears to be due either to a tendency to overestimate cognitive difficulties by self-report where objective measures find no significant issue or a failure to report cognitive dysfunction where objective measures would suggest significant difficulties. Importantly, most studies do find increased objective cognitive difficulties in individuals posttreatment but report little overlap with subjective reports.
As an example, following on their observation of patient reports of cognitive difficulties described above, Cull et al. (2) found no significant difference in performance on objective memory or concentration tasks between those reporting cognitive difficulties and those reporting no issues. Similarly, Schagen et al. (3) found no relationship between the cognitive reports and objective cognitive performance of breast cancer survivors treated with CMF, despite a higher rate of objective cognitive difficulties in the CMF-exposed group. This same inconsistency between self-report and objective measures is found across several later studies and in those described above, with studies either finding no relationship between subjective and objective measures or that subjective reports were better accounted for by other variables.
The source of this discrepancy has received increasing attention as these inconsistencies have come to light (for review, see Ref. 25) and is important to understand when assessing the reports of patients’ post-treatment. While objective findings of cognitive dysfunction have been documented in previous studies, it is important to note that use of these objective measures either clinically or in research applications may tend to underestimate cognitive dysfunction. To what extent these measures are actually predictive of day-today functioning, that is, their ecological validity is not clear; to what extent neuropsychological measures are adequately sensitive to what are likely subtle deficits is also in question. Additionally, formal assessments typically take place in a somewhat rarefied environment that includes no distractions and no competing demands in a quiet, generally predictable setting, all important factors for individuals who complain of attentional difficulties and distractibility. In the research context, in addition to being subject to all of the caveats described in the clinical setting, prior research has excluded individuals who may be at most risk for cognitive dysfunction because of a history of neurological, medical, or psychological difficulties that may in fact act as risk factors to treatmentrelated effects. As Pullens et al. (25) note, several factors may be in play: 1) The sensitivity of objective measures may be questioned in their ability to identify what may be subtle cognitive deficits; 2) objective measures may fail to measure the kinds of difficulties that most affect patients post-treatment; 3) patients may be describing their worst functioning over a period of months versus a one-time, cross-sectional objective measurement; 4) increasing patient exposure and knowledge of “chemobrain” may lead to increased reporting; 5) subjective reports may reflect poor-self evaluation as a result of depression, anxiety, or stress. Emotional factors, in particular, have been found to be associated with increased reporting of cognitive decline post-treatment in the absence of objective decline. Van Dam et al. (13) found a higher incidence of objective cognitive difficulties in high versus low dose and healthy control patients as well as a higher incidence of reported cognitive difficulties between chemotherapy-treated patients and healthy control participants. Significantly, while objective performance and subjective reports were not associated, subjective reports were associated with elevated scores on the anxiety and depression subscales of the Hopkins Symptom Checklist, with similar findings in a subset of other studies (3,7,12,26,27). Subjective expectation of cognitive difficulties may also affect increased self-reporting as a result of increasing awareness of treatment-related effects. This is suggested by Schagen et al. (28), who found that, similar to other schema induction research (29,30), individuals who had preexisting knowledge of potential treatment effects reported more cognitive difficulties.
Despite this disagreement between subjective and objective measures, findings of objective cognitive decline posttreatment are replete in the literature. Objective measures provide what may be considered the most conservative estimate of cognitive decline. The limitations of objective measures, including potentially poor sensitivity, poor ecological validity, and differences between the testing environment and real-world settings, however, may tend to underestimate actual cognitive dysfunction in cancer patients post-treatment.
RISK FACTORS AND MODERATORS OF COGNITIVE CHANGE FOLLOWING TREATMENT
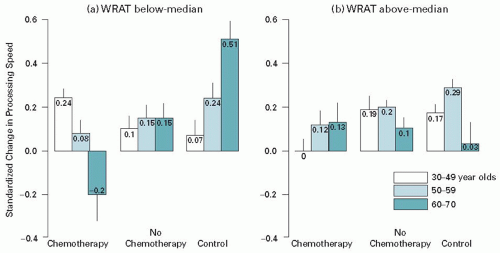
In light of previous research finding pre-treatment differences in a subset of cancer-diagnosed individuals together with the more recent research that suggests only a subset of individuals experience cognitive decline following treatment, a next logical step is to examine risk factors that increase vulnerability to cognitive change and factors that may compensate for, or confer resilience to, these changes. Cognitive changes may be understood as the product of intrinsic factors—age appropriate and pathological changes in structural and functional integrity in the brain—together with what may be broadly characterized as environmental exposures, either currently or by history, that may be either protective or represent a risk factor to cognitive functioning. Work on the concept of brain reserve, that is, the theory that structural integrity or burden in the brain can influence the course of cognitive function in the face of new onset insults, has realized increasing attention in studies of dementing conditions and traumatic brain insult (31). This work has mainly focused on resilience to cognitive deterioration in conditions in which progressive decline is a natural course (Alzheimer’s disease, frontotemporal dementia, Lewy body disease) (32) (for review, see Ref. 33). The concept of cognitive reserve, that is, innate and developed cognitive capacity which is influenced by various factors including genetics, education, occupational attainment, and life style, is related to brain reserve but is defined as a more active, compensatory process (34). Age, premorbid cognitive ability, and genetic inheritance have all been identified as significant factors that may moderate or mediate cognitive difficulties following treatment. Age is a well-established risk factor for cognitive decline in other disorders; researchers have speculated that older adults may be more vulnerable to a variety of insults partly as a result of decreased brain reserve mediated by brain pathology and the accumulated effects of age-appropriate changes in brain structure (35). Individuals with lesser cognitive abilities pre-treatment, which may be indicative of lesser cognitive reserve, may also be at greater risk for post-treatment cognitive changes. Research has demonstrated that people with low cognitive reserve are more vulnerable to the development of neurocognitive disorders (Alzheimer’s disease) and to cognitive decline following a variety of insults to the brain. Further, research has demonstrated poorer cognitive outcomes secondary to neurotoxic exposures (e.g., lead) in people with low cognitive reserve (36). Based on the concepts of brain and cognitive reserve, one would predict that older patients with lower than expected cognitive performance at pre-treatment would demonstrate poorer cognitive performance post-treatment. Ahles and colleagues found support for an interaction of age, cognitive reserve, and exposure to chemotherapy as risk factors for cognitive decline. In the context of a longitudinal study, they demonstrated that older patients who had lower levels of estimated premorbid function demonstrated significantly reduced performance on posttreatment measures of processing speed (see Figure 43.1) (37).
Genetic factors have also been examined as potential risk factors for cognitive decline. Apolipoprotein E (ApoE) is a complex glycolipoprotein that facilitates the uptake, transport, and distribution of lipids. It appears to play an important role in neuronal repair and plasticity after injury. A four exon gene codes for ApoE on chromosome 19 in humans. There are three major alleles: E2, E3, and E4. These alleles differ in amino acids at positions 112 and 158: E2 (cysteine/cysteine), E3 (cysteine/arginine), and E4 (arginine/arginine). Animal models suggest a link between the E4 allele and increased mortality, extent of damage, and poor repair following trauma (38). The human E4 allele has been associated with a variety of disorders with prominent cognitive dysfunction including healthy individuals with memory difficulties,
Alzheimer’s disease, and poor outcomes in stroke and traumatic brain injury. Ahles et al. (39) evaluated the relationship between the ApoE genotype and neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. The results demonstrated that survivors with at least one E4 allele scored significantly lower in the visual memory and spatial ability domains, with a trend to score lower in the psychomotor domain, compared with survivors who did not carry an E4 allele.
Alzheimer’s disease, and poor outcomes in stroke and traumatic brain injury. Ahles et al. (39) evaluated the relationship between the ApoE genotype and neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. The results demonstrated that survivors with at least one E4 allele scored significantly lower in the visual memory and spatial ability domains, with a trend to score lower in the psychomotor domain, compared with survivors who did not carry an E4 allele.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree