Clostridioides (Clostridium) difficile
Larry K. Kociolek
Erik R. Dubberke
Clostridioides difficile (formerly Clostridium difficile) was originally identified in the stool of a healthy, asymptomatic infant in 1935 (and was originally called Bacillus difficilis),1 but more than 40 years elapsed before C difficile was identified as the etiologic agent of antibiotic-associated pseudomembranous colitis (PMC) in 1978.2 Since that time, C difficile has emerged as the most important identifiable cause of healthcare-associated infectious diarrhea. C difficile is now the most common healthcare-associated pathogen in the United States,3 causing ˜500 000 infections and 29 000 deaths in the United States each year.4 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified C difficile as an “urgent” public health threat that requires “urgent and aggressive action”.5 This call to action has led to new discoveries that have advanced C difficile infection (CDI) epidemiology, diagnosis, treatment, and prevention.
PATHOGENESIS AND CLINICAL DISEASE SPECTRUM
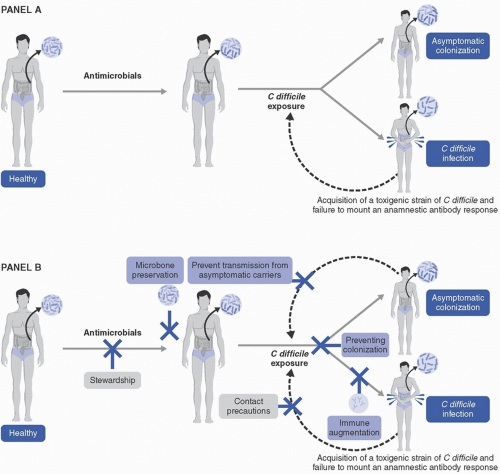
Increased understanding of the pathogenesis of CDI has guided the development of strategies for diagnosing, preventing, and treating C difficile. C difficile is an anaerobic Gram-positive rod. Strains that possess genes for production of toxins are considered toxigenic; nontoxigenic strains lack these genes and are considered nonpathogenic. The pathogenesis of CDI is multifactorial and complex. The series of events leading to CDI, and potential strategies for prevention, are shown in Figure 17-1.
CDI occurs in a susceptible host following exposure to spores from toxigenic strains of C difficile, but exposure alone is insufficient to cause CDI. C difficile spores are well suited to germinate in a dysbiotic intestinal environment, which often results from receipt of antibiotics shortly before or after C difficile exposure. Virtually any antibiotic is able to cause dysbiosis and increase the likelihood of developing CDI. Historically, C difficile strains were commonly resistant to clindamycin, which contributed to the well-known association of clindamycin and PMC. However, since that time, the emergence of fluoroquinolone resistance in epidemic strain ribotype (RT) 027 led to the recognition of fluoroquinolones as a major risk factor for CDI.6 Further, with the expanded use of broad-spectrum cephalosporins, and because of intrinsic cephalosporin resistance in C difficile, cephalosporins are also considered a major contributor to CDI.7
After C difficile spores germinate in the gut, vegetative cells express toxins that are cytocidal to colonocytes and initiate an inflammatory cascade that lead to CDI symptoms, most commonly a mild-to-moderate diarrheal illness (described in more detail below). While the importance of both toxins A (an enterotoxin) and B (a cytotoxin) have been described,6 it is currently believed that toxin B is the primary contributor to CDI based on animal models of infection8 and the observation of CDI caused by strains lacking toxin A.9 These findings are supported by recent data demonstrating lower risk of recurrent CDI in patients with high anti-toxin B antibody titers, while anti-toxin A antibody titers did not impact CDI recurrence risk.10 Some C difficile strains additionally express binary toxin, of which the specific role in CDI pathogenesis is not entirely clear.6 Interestingly, for reasons that are poorly understood, infants seem to be innately protected from clinical CDI despite frequent rates of colonization and toxin detection in the stool.11 Based on animal data, this phenomenon is thought to be potentially related to lack of toxin receptor expression in infants,12 but this is unproven in humans. The most common clinical manifestation of CDI is mild-to-moderate diarrhea, but the full spectrum of disease ranges from asymptomatic colonization or fecal excretion to mild-to-moderate diarrhea to fulminant colitis. Signs of fulminant colitis, which is associated with mortality, include CDI associated with hypotension or shock, toxic megacolon, or ileus.7 The association of ileus with fulminant CDI is important to note because diarrhea may be absent and CDI may not be considered in the differential diagnosis.
Sustained cure from CDI is more likely to occur with normalization of the intestinal microbiome and with a humoral immune response against C difficile toxins, particularly against toxin B.10,13 These important observations contributed to the rationale for development of biologic and immunologic strategies for CDI prevention, which are described in more detail below.
EPIDEMIOLOGY
CDI Surveillance Definitions
CDI surveillance is an important hospital infection control and public health activity. CDIs are classified according to history of prior CDIs and location of symptom onset (Fig. 17-2).7 Of note, these definitions are revised from time
to time and may vary somewhat among professional organizations. In general, an incident CDI is defined as a firsttime CDI or CDI that has occurred more than 8 weeks after a prior CDI. Recurrent CDIs are those that occur within 8 weeks from a prior CDI. A positive C difficile test within 2 weeks from a prior CDI is considered a duplicate test, that is, a positive test related to the most recent CDI and generally not indicative of a subsequent CDI. However, with recent guidance limiting CDI treatment to 10 days,7 it is possible for a CDI recurrence to manifest within 10-14 days after the onset of a prior CDI.
to time and may vary somewhat among professional organizations. In general, an incident CDI is defined as a firsttime CDI or CDI that has occurred more than 8 weeks after a prior CDI. Recurrent CDIs are those that occur within 8 weeks from a prior CDI. A positive C difficile test within 2 weeks from a prior CDI is considered a duplicate test, that is, a positive test related to the most recent CDI and generally not indicative of a subsequent CDI. However, with recent guidance limiting CDI treatment to 10 days,7 it is possible for a CDI recurrence to manifest within 10-14 days after the onset of a prior CDI.
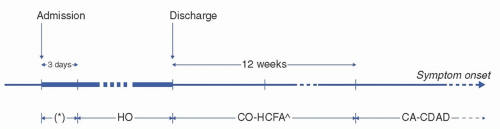 FIGURE 17-2 Surveillance definitions for C difficile infection (CDI) exposures.14 Healthcare facility-onset (HO) CDIs are those with onset more than 3 days after hospital admission. Case patients with symptom onset during the first 3 days of hospitalization marked by an asterisk (*) would be classified as having community-onset healthcare facility-associated (CO-HCFA) if CDI occurred in a patient who was recently admitted overnight to a healthcare facility in the past 12 weeks or community-associated (CA) if CDI occurred in a patient not admitted overnight to a healthcare facility in the past 12 weeks. ˆAdditional surveillance guidance delineates CO-HCFA CDIs into indeterminate CDIs if the patient was recently admitted overnight to a healthcare facility in the past 4-12 weeks.15 (Adapted from McDonald LC, Coignard B, Dubberke E, et al. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007;28:140-145. Ref. 16.) |
Incident CDIs can be distinguished further based on location of onset and prior hospital admission chronology. Per the CDC Emerging Infections Program,14 healthcare facility-onset (HO) CDIs are those with onset more than 3 days after hospital admission. Non-HO CDIs are broadly referred to as community-onset (CO) and further delineated based on history of hospital admission. CO-healthcare facility-associated (CO-HCFA) CDIs are those with onset in the community or within the first 3 days of hospitalization in a patient who was recently admitted overnight to a healthcare facility in the past 12 weeks. Additional surveillance guidance from the Society for Healthcare Epidemiology of America (SHEA) further delineates CO-HCFA CDIs into indeterminate CDIs if the patient was recently admitted overnight to a healthcare facility in the past 4-12 weeks.15 Together, HO- and CO-HCFA CDIs are often referred to collectively as healthcare-associated CDIs. Finally, communityassociated (CA) CDIs are those with onset in the community or within the first 3 days of hospitalization in a patient who was not admitted overnight to a healthcare facility in the past 12 weeks.
Epidemic Characteristics
Prior to the early 2000s, CDI surveillance was not routinely performed; however, outbreaks of particular strain types were reported to occur. For example, limited retrospective molecular epidemiology data suggested that restriction endonuclease analysis group B predominated in the 1980s.17 An outbreak of RT001 (also known as restriction endonuclease analysis group J) occurred in the United States between 1989 and 1992.18 In the early 2000s, CDI rates were observed to increase in the United States and Canada. While this epidemic mainly affected older adults, CDI rates also increased among younger populations who were previously considered low-risk populations.6 For example, in 2005, reports of severe CDI among patients in the community without exposure to healthcare facilities (sometimes without antibiotic exposure) and reports of CDI in peripartum women and children highlighted the concern that the epidemiology of CDI was expanding beyond traditional risk populations.19 Today, CA CDI is a well-described phenomenon. CA CDI accounts for approximately one-third of all CDIs among adults4 and more than two-thirds of all CDIs among children.20
This increased incidence was also associated with increased CDI severity and mortality. This epidemic was ultimately determined to be caused by the emergence of an epidemic strain, known as RT027, which had virulence factors distinct from prior epidemic strains, such as those previously described. These include binary toxin production, a loss-of-function mutation in the tcdC-negative repressor of toxins A and B (potentially leading to increased production of toxins A and B, although this is controversial), and fluoroquinolone resistance. It is thought that these features contributed to the widespread dissemination of this strain and its association with more severe disease phenotypes. With urgent and aggressive action to limit spread of this multidrug-resistant C difficile strain, RT027 incidence has declined in the United States. For example, RT027 accounted for 17% and 21% of CA- and healthcare-associated CDIs in 2012, respectively, but in 2017, RT027 only accounted for 6% and 15% of CA- and healthcare-associated CDIs in 2012, respectively. Nonetheless, RT027 remains the most common strain type of C difficile in healthcare facilities. However, RT106 has surpassed RT027 as the most common US strain type causing CA CDI and total CDI.21
Current US CDI Burden
The burden of CDI in the United States has been well characterized by the CDC Emerging Infections Program, which is a 10-state collaborative that performs active population laboratory-based CDI surveillance. According to the 2015 publication highlighting surveillance data collected in 2011, the US pooled mean crude incidence rates of CA CDI and healthcare-associated CDI were 48 and 93 cases per 100 000 persons, respectively, for an overall adjusted national CDI incidence of 147 cases per 100 000 persons.4 CDI rates varied from state to state with incidence rates of CA-CDI and healthcare-associated CDI ranging from 27 to 124 and 48 to 159 cases per 100 000 persons, respectively. CDI incidence rates are higher among women and white race, and incidence increases with age with adults older than 65 years having an incidence rate more than fourfold higher than those between 45 and 64 years old. The percentage of cases of healthcare-associated CDIs with symptom onset in acute care hospitals, nursing homes, and the community was ˜37%, 36%, and 28%, respectively. Overall, these data suggest that more than 450 000 incident CDI cases and 29 000 CDI-associated deaths (ie, crude mortality within 30 days of CDI diagnosis) occur in the United States annually.
CDI Outcomes
CDI recurrences are a major contributor to CDI morbidity. It is estimated that ˜14% and 21% of patients with CA-and healthcare-associated CDI, respectively, experience a CDI recurrence within 8 weeks,4 with risk of recurrence doubling after a first recurrence.22 Independent risk factors for recurrence include age >65 years, fluoroquinolone exposure, additional systemic antibiotics after CDI, proton pump inhibitor use, and renal insufficiency.22 Recovery from dysbiosis after CDI13 and development of a humoral immune response against C difficile toxin B10 reduce the likelihood of CDI recurrence.
While most cases of CDI are mild-to-moderate diarrheal illnesses, severe (associated with leukocytosis or acute kidney injury) and/or fulminant CDIs (associated with hypotension, ileus, or toxic megacolon) occur commonly. Fulminant CDI requiring colectomy, which occurs with ˜1% of CDIs during endemic periods and up to 6% of CDIs during epidemic periods,7 is a major contributor to mortality. Death within 30 days follows ˜1% and 9% of CA- and healthcare-associated CDIs, respectively. Risk factors
for fulminant CDI include clindamycin or proton pump inhibitor exposure,23 and serum albumin and presence of renal disease predict a fulminant course.24 As previously noted, infection with RT027, the acquisition of which is associated with fluoroquinolone exposure, also leads to more severe outcomes.6
for fulminant CDI include clindamycin or proton pump inhibitor exposure,23 and serum albumin and presence of renal disease predict a fulminant course.24 As previously noted, infection with RT027, the acquisition of which is associated with fluoroquinolone exposure, also leads to more severe outcomes.6
Antimicrobial Use
Nearly every antibacterial agent given by either oral or parenteral routes has been associated with CDI. Historically, the most commonly implicated agent had been clindamycin,25 but the importance of other antibiotics, including broad-spectrum cephalosporins and carbapenems, is well described.26 As described above, since 2000, fluoroquinolones have been recognized as a class of antimicrobials with a particularly high risk of CDI.6 Fluoroquinolones were the most frequently implicated antimicrobial associated with CDI during multihospital outbreaks of RT027 in Canada27 and the United States.28 These data suggested that increasing fluoroquinolone use had facilitated dissemination of the once uncommon RT027 strain that developed high-level fluoroquinolone resistance.
Reservoirs and Modes of Transmission
With the emergence of exquisitely sensitive whole genome sequencing (WGS) to track C difficile, much has been learned about patient-to-patient transmission of C difficile. In a large hospital-based study in the United Kingdom, only 35% of new CDIs could be linked to another symptomatic patient with CDI.29 In children, other symptomatic patients are an even less common source of infection; only 10% of HO CDIs could be linked to another symptomatic patient, although use of polymerase chain reaction (PCR) for C difficile diagnosis may have biased these findings by including both colonized and infected children.30 These studies suggest the presence of other common reservoirs of infection, such as community reservoirs or asymptomatic C difficile carriers in healthcare settings. Rigorous molecular epidemiologic investigation using WGS suggests that while carriers do transmit C difficile, they transmit less frequently than symptomatic patients. In a multihospital Canadian study, 14% of CDIs could be linked to a patient with CDI, whereas only 6% of CDIs could be linked to asymptomatic carriers.31 Multiple studies in the United Kingdom similarly reported low frequency of transmission from asymptomatic carriers.32,33
Environmental surfaces contaminated with C difficile spores are a potential source of C difficile transmission, which could lead to healthcare-associated CDIs. The environment of patients with CDI and asymptomatic C difficile carriage34 is more frequently contaminated than the environment of other patients, and the degree of contamination has correlated with C difficile outbreaks.35,36,37 Floors and bathroom sites tend to be most heavily contaminated.38 In addition, commode chairs, sigmoidoscopes, bed pans, nursery baby baths, patient phones, and electronic thermometers have been found to be contaminated and can serve as reservoirs for healthcare-associated transmission of C difficile.39,40,41
If either the environment or asymptomatic carriers are important sources of infections, C difficile could be transmitted from those sources by direct contact or indirectly by the hands or gloves of patient care personnel. Hands35,36,42 are frequently contaminated with C difficile, and hand colonization rates as high as 59% after contact with a patient with CDI when gloves were not worn, which, in some instances, amounted to mere patient assessment and charting, have been documented.35 Vinyl glove use by hospital personnel when handling body substances was also associated with a significant reduction in the incidence of CDI on acute care wards.43 Thus, direct and indirect evidence supports transient hand carriage by patient care personnel as a mode of C difficile transmission.
Several studies performed in many different countries provide clinical and molecular epidemiologic evidence of potential community reservoirs for CDI, including food and animal sources. These reservoirs may be strain specific. In Europe, particular RTs, such as RT027, are associated with country- and hospital-based clustering, implicating the role of the healthcare environment in transmission. On the other hand, other RTs, including RT078, RT014, and RT020, do not demonstrate country- and hospital-based clustering, suggesting the existence of widely disseminated community reservoirs.44 Interestingly, these RTs have also been identified in animals45,46 and food products,47 and genetic linkages between community reservoirs and human clinical C difficile isolates have been established.46
DIAGNOSIS
CDI diagnosis is a significant challenge and a major clinical conundrum. Here, we review important infection prevention and surveillance related aspects of C difficile diagnostics. Readers are referred elsewhere for a more comprehensive review of C difficile diagnostics48 and clinical recommendations regarding their use jointly from the Infectious Diseases Society of America (IDSA) and SHEA.7 There are several different types of C difficile diagnostic tests that vary based on ease of performance, turnaround time, sensitivity, and diagnostic and analytical predictive value.48,49 In general, diagnostic tests for detecting toxigenic strains of C difficile in the stool can be broadly categorized as either tests that detect secreted C difficile toxins or tests that detect C difficile strains that have the potential to produce toxin. Diagnostic tests can be utilized individually or as part of a multistep algorithm, such as those incorporating a screening test such as enzyme immunoassay (EIA) for glutamate dehydrogenase (GDH). GDH is a sensitive assay to detect an antigen produced by both toxigenic and nontoxigenic strains of C difficile. Thus, a positive GDH test should be followed up with an assay to confirm the presence of C difficile toxins or toxigenic C difficile strains in stool.
Free toxin is typically measured by a cell culture cytotoxicity neutralization assay (CCNA) or EIA.48 CCNA, the gold standard for toxin detection, is a labor-intensive assay that takes several days to result. Cell cultures are exposed to a fecal filtrate with and without first incubating the fecal filtrate with antibodies that neutralize toxins A and B. Free toxin is detected if cytotoxicity (ie, cell rounding) is noted in the well containing the fecal filtrate but not the well with the fecal filtrate that was first incubated with neutralizing antitoxin antibodies. Of note, although considered the gold standard for toxin testing, CCNA is not standardized
because of variability in utilized cell lines, neutralizing antibodies, and negative controls, in addition to interobserver variability in identification of cell cytotoxicity. Thus, CCNA is rarely used clinically. Toxin EIA is the most commonly used toxin detection test in the clinical setting, and many commercially available EIAs are inexpensive and can provide a result in less than an hour.
because of variability in utilized cell lines, neutralizing antibodies, and negative controls, in addition to interobserver variability in identification of cell cytotoxicity. Thus, CCNA is rarely used clinically. Toxin EIA is the most commonly used toxin detection test in the clinical setting, and many commercially available EIAs are inexpensive and can provide a result in less than an hour.
To detect toxigenic C difficile strains (ie, not the secreted toxin) in stool, toxigenic stool culture is the gold standard. Stool is first cultured anaerobically for C difficile.48 Because toxigenic and nontoxigenic strains can both be cultured from stool, C difficile isolated from stool is then grown in broth media and tested for toxin production in vitro. Similar to CCNA, toxigenic stool culture is labor intensive, takes several days to result, and is rarely used clinically in the United States. Nucleic acid amplification tests (NAATs), such as PCR and loop-mediated isothermal amplification (LAMP) tests, detect tcdA and/or tcdB (ie, the genes for toxins A and B, respectively). Thus, NAATs detect the machinery necessary to produce toxin in vivo but do not determine whether toxin was actively being expressed in the gut at the time of the test.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree