Reference
Regimen
Patients (no.)
Median age (years)
ORR (%)
CR (%)
OS
Kantarjian et al. [19]
Clofarabine monotherapy
112
71
46
38
41 weeks
Faderl et al. [26]a
Clofarabine + cytarabine
70
71
31–63
31–63
5.8–11.4 months
Burnett et al. [20]
Clofarabine monotherapy
406
74
38
22
12%b
Kadia et al. [25]c
Clofarabine + cytarabine
118
74
68
60
11.1 and18.5 months
Foran et al. [21]
Clofarabine monotherapy
727
68
42.8
42.8
1.41d
A large randomized ECOG trial established that clofarabine does not increase survival for older patients with de novo or secondary AML [20]. Burnett et al. compared clofarabine and low-dose cytarabine (LDAC), a common treatment for induction in elderly patients unable to tolerate 7 + 3, in 406 patients (median age of 74 years) with newly diagnosed de novo AML, secondary AML, or high-risk MDS. The ORR was 38% in the clofarabine arm compared to 19% in the LDAC arm, and 2-year survival was 13% for clofarabine compared to 12% for LDAC. While there was no difference in OS, relapse-free survival was 20% for the clofarabine arm compared to 8% for the LDAC arm.
Clofarabine monotherapy was unable to improve survival compared to 7 + 3 in a recent trial sponsored by ECOG. A total of 727 patients ≥60 years of age and newly diagnosed with AML were randomized to clofarabine monotherapy or 7 + 3 with the option of decitabine maintenance following induction. Preliminary results presented at the American Society of Hematology Annual Meeting in 2015 revealed no significant difference in OS, with a hazard ratio (HR) of 1.41 [21]. Furthermore, CR rates were nearly identical: 42.8% for the clofarabine arm and 43.8% in the 7 + 3 arm.
17.2.1.2 Clofarabine/Cytarabine Combination Therapy in Adults with AML
The promising responses observed with clofarabine monotherapy in hematologic malignancies led to the studies of clofarabine in combination with other cytotoxic agents in patients with AML. FLAG [i.e., fludarabine and cytarabine with granulocyte-colony stimulating factor (G-CSF) priming] is a common regimen used for patients with relapsed AML and has been in use since the 1990s. Becker et al. substituted clofarabine for fludarabine, based on their structural similarity and the higher response rates achieved with clofarabine [22]. The study evaluated 46 patients with refractory/relapsed AML and a median age of 53 years and demonstrated an ORR of 61% with CR rate of 46%. The authors suggested that this combination therapy could be a viable option for this patient population given the lack of dose-limiting toxicity (DLT; Table 17.2).
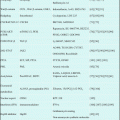
Table 17.2
Clofarabine studies in adults with relapsed AML
Reference | Regimen | Patients (no.) | Median age (years) | ORR (%) | CR (%) | OS (months) |
|---|---|---|---|---|---|---|
Faderl et al. [28]a | Clofarabine + cytarabine | 25 | 59 | 42 | 25 | 7.9 |
Faderl et al. [26]b | Clofarabine + idarubicin + cytarabine | 44 | 56–58 | 48 | 48 | Not reported |
Becker et al. [22] | Clofarabine + cytarabine | 46 | 53 | 61 | 46 | Not reported |
Faderl et al. [23] | Clofarabine + cytarabine | 320 | 67 | 46.9 | 35.2 | 6.6 |
In 2012, the CLASSIC I trial was conducted as a follow-up to the phase 1/2 study that had established the activity of clofarabine in relapsed AML [23]. This phase 3 trial was a double-blind, placebo-controlled study that compared clofarabine combined with cytarabine to cytarabine and placebo in patients ≥55 years of age with relapsed or refractory AML. A total of 320 patients with a median age of 67 were evaluated. Patients in the clofarabine + cytarabine arm had a better ORR than cytarabine + placebo (46.9% vs. 22.9%). Clofarabine also had a 37.7% 4-month event-free survival (EFS) compared to 16.6% with placebo; however, there was no significant difference in the median OS between clofarabine and placebo (6.6 vs. 6.3 months, P = 1.00).
Similar results were seen when 5 days of clofarabine monotherapy at 30 mg/m2 was compared to clofarabine + LDAC dosed subcutaneously for 14 days at 20 mg/m2 [24]. In this study, 70 patients ≥60 years of age with AML or high-risk MDS were randomized. The median age of the patients was 71 years. Clofarabine combined with LDAC had a CR rate of 63% with an induction mortality of 19%, whereas clofarabine monotherapy had a CR rate of 31% with an induction mortality of 31%. OS was equivalent between clofarabine combined with LDAC and clofarabine monotherapy (11.4 vs. 5.8 months; P = 0.10). In another study, Kadia et al. evaluated 118 patients with a median age of 74 and newly diagnosed AML and treated with clofarabine and LDAC followed by alternating cycles of decitabine maintenance/consolidation [25]. Though there was no comparator arm, the ORR was 68% with a 60% CR rate, which was higher than any rates seen in any prior induction AML clofarabine trials.
17.2.1.3 Clofarabine/Anthracycline Combination Therapy in Adults with AML
A phase 1 study investigated the efficacy of clofarabine in 44 patients ≥18 years of age in the first relapse or with primary refractory AML [26]. Patients were randomized to either clofarabine and idarubicin or clofarabine, idarubicin, and cytarabine (CIA). The maximum tolerated dose (MTD) in the clofarabine/idarubicin arm was clofarabine 22.5 mg/m2 IV daily for 5 days and idarubicin 10 mg/m2 IV daily for 3 days. The MTD for CIA was the same, with the addition of cytarabine 0.75/m2 IV for 5 days. DLTs were hyperbilirubinemia, hepatic transaminitis, mucositis, and diarrhea. Though it was not a primary end point of the study, a response rate of 48% was seen.
This study was followed by a phase 2 study of CIA in patients with newly diagnosed AML 60 years of age or younger [27]. Induction therapy consisted of clofarabine given at a dose of 20 mg/m2 for 5 days, idarubicin given at a dose of 10 mg/m2 on days 1–3, and cytarabine given at a dose of 1 g/m2 on days 1–5. Patients in remission were consolidated with up to six cycles of CIA given on an attenuated dosing schedule. Of the 57 patients evaluable, an ORR of 79% was attained, with the median OS not yet reached after a median follow-up of 10.9 months. Median EFS was 13.5 months. Therapy was well tolerated with 4-week mortality rate of 2%. OS and EFS of patients treated with CIA compared favorably to historical controls treated with 7 + 3, even after multivariate analysis.
17.2.2 Myelodysplastic Syndrome (MDS)
In addition to activity in AML, the previously discussed study by Faderl et al. also demonstrated clofarabine activity in patients with MDS [28]. In a small patient population, a CR rate of 25% was achieved in high-risk MDS patients. Subsequent studies looked at oral dosing of clofarabine and higher-risk MDS (Table 17.3). Thirty-two patients with intermediate- and high-risk disease based on the International Prognostic Scoring System were evaluated [29]. Nearly all of the patients had secondary MDS or were refractory to hypomethylating agents (HMA). Despite the population having a median age of 70 years, an ORR of 43% was seen, with 25% achieving complete remission. Toxicity increased at higher doses. Of note, 16% of patients experienced acute renal failure. Hepatic and gastrointestinal adverse events (AEs) were seen at all doses. The toxicity profile was studied further in a phase 2 trial; the results of which were reported in 2012. Fifty-eight patients with a median age of 68 years and higher-risk MDS were adaptively randomized to lower- and higher-dose treatment arms [30]. The side effect profile was similar for both arms and consistent with prior reports but more severe at the higher-dose level. The ORR was 36% with a CR rate of 26%. Despite the response rate seen in these trials, the majority of high-risk MDS patients will fail all available therapy or relapse quickly after response [31]. One hundred and nine patients with MDS or chronic myelomonocytic leukemia (CMML) and a median age of 67 years were evaluated. Of the patients evaluated, 38 had received clofarabine as first-line therapy, 59 received it after failing an HMA, and 22 had failed 2 or more MDS-directed therapies. Following failure, the OS in this patient population was 4.3 months, which correlates closely with OS post-HMA failure [31, 32]. Given that survival for high-risk MDS patients is between 11 and 13 months after clofarabine therapy, there remains significant opportunity to research and improve the outcomes of these patients [30, 31].
Table 17.3
Clofarabine studies in adults with higher-risk MDS
Study | Regimen | Patients (no.) | Median age (years) | ORR (%) | CR (%) | OS (months) |
|---|---|---|---|---|---|---|
Faderl et al. [28]a | Clofarabine + cytarabine | 25 | 59 | 42 | 25 | 7.9 |
Faderl et al. [29]b | Oral clofarabine | 32 | 70 | 43 | 25 | 9.2 and 13.8 |
Faderl et al. [30]c | Clofarabine | 58 | 68 | 36 | 26 | 7.4, 13.4, and 21.7 |
Ghanem et al. [31]d | Clofarabine | 109 | 67 | Not evaluated | Not evaluated | 4.3 |
17.2.3 Acute Lymphoblastic Leukemia (ALL)
Two Southwest Oncology Group (SWOG) studies, led by Advani and colleagues, explored the use of clofarabine in adult ALL. In the first study, 37 patients were treated with clofarabine 40 mg/m2/day and cytarabine 1 g/m2/day on days 1–5 [33]. The median age was 41 years; 44% of patients were in a second or subsequent relapse or had refractory disease and 59% of patients had high-risk cytogenetics. Six out of thirty-six patients (17%) achieved a complete remission with or without complete count recovery. Median OS was 3 months. In the second study, SWOG study S0910, epratuzumab, a humanized monoclonal antibody against CD22, was combined with clofarabine and cytarabine in adults with relapsed/refractory pre-B ALL [34]. The complete remission rate with or without complete count recovery with the addition of epratuzumab was 52%.
Faderl and colleagues treated 50 patients with a median age of 30 years with clofarabine 40 mg/m2 daily for 3 days and cyclophosphamide 200 mg/m2 every 12 h for 3 days [35]. The response rate of the whole study group was 14%, including 10% of patients who achieved CR or CR without hematologic recovery. Three responses occurred in patients with primary refractory disease. The early mortality rate (<30 days) was 6%. The median duration of response was 69 days, and the median OS was 3 months. Another retrospective study of 55 patients treated with clofarabine combination therapy showed a 50% remission rate in relapsed/refractory patients, with 17–35% of patients proceeding to alloSCT [36].
17.3 Clofarabine for Pediatric Leukemia
Since the first phase 1 study of clofarabine was initiated in 1993 in adult patients with hematologic and solid malignancies [37], several clinical trials have demonstrated the clinical activity of clofarabine monotherapy for hematological malignancies. Moreover, combination regimens have been tested that leverage the pharmacological properties of clofarabine by combining it with traditional antileukemic drugs, revealing promising results [38–40]. Development of new therapeutic agents for children has historically lagged behind their introduction in adults. Most published studies of clofarabine have been conducted in adult patients with AML or MDS. However, unlike its predecessors, clofarabine was approved by the FDA in 2004 specifically for use in pediatric patients with relapsed or refractory ALL who have failed treatment with at least two prior regimens.
17.3.1 Clofarabine in Pediatric Acute Lymphoblastic Leukemia (ALL)
With contemporary first-line chemotherapy regimens, the vast majority of newly diagnosed pediatric patients with ALL will achieve remission, and the OS rate is approaching 90% [41]. Despite our overall success in treating newly diagnosed ALL, long-term cure rates for children who relapse remain poor, and relapsed ALL is still the leading cause of death among pediatric oncology patients [41]. The FDA approval of clofarabine was based on phase 1 and 2 studies in pediatric patients with refractory or relapsed ALL in which clofarabine monotherapy had demonstrated activity [42]. Based on the promising monotherapy data, several combinations of clofarabine with other agents were subsequently evaluated in pediatric patients with relapsed ALL [43–46].
17.3.1.1 Clofarabine Monotherapy in Pediatric ALL
The initial phase 1 study of clofarabine monotherapy for children with ALL was done at MD Anderson Cancer Center in conjunction with a study in adults [15, 47] (Table 17.4). Twenty-five children with ALL (n = 8) or AML (n = 17) received six dose levels between 11.25 and 70 mg/m2 IV over 1 h for 5 consecutive days. Five patients (one AML and four ALL) achieved CR, and three patients (two AML and one ALL) achieved a partial response (PR), yielding an ORR of 32%. Four out of 17 children (24%) with ALL and one of eight patients with AML (13%) attained CR. The DLTs were reversible hepatotoxicity and skin rash, which were seen at 70 mg/m2/day for 5 days, and the MTD was 52 mg/m2/day for 5 days.
Table 17.4
Published studies of clofarabine in children with ALL
Phase | Combination | References | |
|---|---|---|---|
Phase 1 | CLO | [47] | |
CLO212 | Phase 2 | CLO | [42] |
A report from UK | – | CLO (n = 5) CLO, CTX, VP (n = 18) | [83] |
CLO21800205 | Phase 1/2 | CLO, CTX, VP | |
Italian multicenter study | Phase 2 | CLO, CTX, VP | [46] |
Italian study | – | CLO, CTX, VP (n = 24) | [58] |
COG AALL1131 | Phase 3 | CLO, CTX, VP, PEG, VCR | [57] |
COG AAML0523 | Phase 1/2 | CLO, ara-C | [45] |
POE07–01 | Phase 1 | CLO, CTX | [52] |
VANDEVOL Study | Phase 1 | CLO, Mito, VP, Asp, Dex | [53] |
TVTC protocol | Phase 2 | CLO, Thio, Topo, Vinorel, Dex | [59] |
CoALL trial 08–09 | Phase 2 | CLO, PEG | [84] |
A phase 2 study of clofarabine monotherapy was conducted in several institutions across the US [42]. Sixty-one children with ALL had received a median number of three prior regimens (range 2–6), including prior hematopoietic stem cell transplantation (HSCT) in 30% of the patients, and 35 patients (57%) were refractory to the last salvage regimen received before clofarabine. Patients received clofarabine at the MTD of 52 mg/m2/day for 5 days every 2–6 weeks [42] (Table 17.4). Of the 61 children in the study, seven (12%) attained CR and five (8%) CR without platelet recovery (CRp) for an ORR of 20%; 10% had PR. The remission was durable, and patients were able to proceed to HSCT after receiving clofarabine. Remissions were sustained in patients who did not undergo an HSCT for a median of 6 weeks. Four patients remained in CR or CRp for 8+, 12, 37+, and 48 weeks after receiving only clofarabine. Febrile neutropenia, anorexia, hypotension, and nausea were the most common side effects.
Another phase 2 study of clofarabine monotherapy was conducted in Europe [48]. In this study, 65 pediatric patients with relapsed/refractory ALL were enrolled, and data from the first 53 patients have been presented. In this study, patients received clofarabine also at the MTD of 52 mg/m2 for 5 days. Fourteen of fifty-three (26%) patients who received at least one course of clofarabine had a response: six achieved CR, seven CRp, and one PR. Four patients proceeded to HSCT after receiving clofarabine, and one patient had durable remission for 20+ months. Three patients developed liver toxicity (hyperbilirubinemia, transaminitis).
17.3.1.2 Clofarabine Combination Therapy
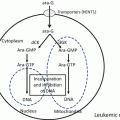
There are a few clofarabine combinations that have been studied based on the mechanism of action of clofarabine. As an inhibitor of ribonucleotide reductase (RnR), clofarabine accumulates cytarabine triphosphate in leukemic cells, thereby increasing the antileukemic activity of cytarabine [40, 49]. Clofarabine also causes depletion of dNTPs, resulting in the inhibition of deoxycytidine kinase through a feedback mechanism. Thus, a combination of clofarabine and cytarabine increases retention of ara-CTP. This combination has been widely investigated in various studies in adult AML [28], pediatric ALL [50], and pediatric AML [51].
Clofarabine inhibits DNA synthesis as well as repair [43]; therefore, combinations of clofarabine and different DNA-damaging agents such as cyclophosphamide [38, 44, 52], epipodophyllotoxins [44, 53], anthracyclines [26], and anthracenedione [53, 54] have also been explored. An in vitro study showed that the cross-links were rapidly repaired in chronic lymphocytic lymphoma (CLL) lymphocytes after exposure to activated cyclophosphamide or 4-hydroperoxycyclophosphamide (4-HC), but DNA repair was impaired when the cells were pretreated with clofarabine [55]. A combination treatment with 4-HC and clofarabine had a synergistic effect on apoptotic cell death, greater than the sum of the effect of each agent [55]. In a phase 1 study of clofarabine and cyclophosphamide in adult patients with leukemia [38], 18 patients received cyclophosphamide (200 mg/m2) alone on day 0 and clofarabine (20 mg/m2 and 10 mg/m2 as dose level 1 [n = 6] and 0 [n = 12], respectively) plus cyclophosphamide on day 1. Prolonged bone marrow suppression was seen as the DLT at dose level 1. Increased DNA damage measured as H2AX phosphorylation was observed in 12 of 13 patients with clofarabine and cyclophosphamide compared with cyclophosphamide alone.
17.3.1.3 Clofarabine Combination Therapy Studies in Pediatric ALL
Clofarabine in Combination with Cyclophosphamide and Etoposide
A combination of clofarabine with cyclophosphamide and etoposide was designed based on evidence of the synergistic effect of clofarabine and DNA-damaging agents [38, 55], as well as previous experience with a regimen of cyclophosphamide and etoposide [56] (Table 17.4). A phase 1/2 study [43, 44] of this combination was conducted. In the phase 1 portion, the primary objectives were to determine the DLT, MTD, and recommended doses for the phase 2 portion of the study [44]. All three drugs were given IV for 5 consecutive days in induction and for 4 consecutive days in consolidation, for a maximum of eight cycles. G-CSF was given to 25 patients (20 with ALL and five with AML) enrolled in 5 cohorts starting 1 day after the last dose of study treatment and continuing until an absolute neutrophil count of 0.75 × 109/L was reached. No DLT was seen in cohorts 1–3. A patient in cohort 4 (clofarabine 30 mg/m2/day, cyclophosphamide 440 mg/m2/day, and etoposide 100 mg/m2/day) had a DLT of grade 3 lipase elevation, grade 3 abdominal pain, and hepatomegaly. Symptoms for which veno-occlusive disease (VOD) could not be excluded occurred in this patient but resolved without any sequelae. Pancreatitis (grade 2) and lipase elevation (grade 3) were observed in another patient in this cohort, but neither met the DLT criteria. This cohort was expanded to ten patients, and no further DLTs occurred. In cohort 5 (clofarabine 40 mg/m2/day, cyclophosphamide 440 mg/m2/day, and etoposide 100 mg/m2/day), a DLT of prolonged bone marrow aplasia beyond day 42 was observed in one patient. Five additional patients were enrolled in cohort 5, and no further DLTs occurred; therefore, MTD was not reached, and the recommended phase 2 doses were determined to be clofarabine 40 mg/m2/day, cyclophosphamide 440 mg/m2/day, and etoposide 100 mg/m2/day for 5 consecutive days. In the phase 1 study, CR was attained in nine patients and CRp in two patients among the 20 patients with ALL. All five patients with AML attained CR (n = 1) or CRp (n = 4). Nine out of sixteen responders proceeded to HSCT.
In the phase 2 portion of the study [43], patients aged 1–21 years with refractory/relapsed ALL were enrolled and received treatment at the recommended dose determined in the phase 1 study (Table 17.4). The primary end point of this study was an overall remission rate (ORR = CR + CRp). Minimal residual disease (MRD) by flow cytometry was evaluated as an exploratory end point. Among the 25 patients enrolled in this study, 14 had two prior induction regimens, seven had three prior induction regimens, and four had one prior induction regimen. Notably, 15 patients (60%) had disease refractory to the immediately preceding regimen and four patients had received a prior HSCT. The ORR was 44%, which comprised seven patients who achieved CR (28%) and four who achieved CRp (16%). In addition, three patients (12%) achieved PR. MRD was evaluable in eight patients. Five of the eight patients were MRD negative (defined as < 0.01%), and three were MRD positive. A total of 10 patients, which included 7 of 11 responders, proceeded to HSCT. The median number of cycles patients received was one (range: 1–3). Ten of eleven responders achieved the best response after one cycle. Treatment-related AEs that occurred in ≥25% of patients were as follows: vomiting (88%); nausea (72%); febrile neutropenia and thrombocytopenia (60% each); anemia (56%); neutropenia and pyrexia (52% each); decreased appetite (44%); ALT increased, AST increased, hypokalemia, and hypotension (36% each); diarrhea (28%); and hyperbilirubinemia (28%). Treatment was discontinued for one patient because of fungal sinusitis, which occurred 17 days after the last dose of study drug. Eighty-eight percent of patients had serious AEs, regardless of relationship to study drug. It should be noted that six patients (24%) died within 30 days of the last dose of study treatment; these deaths were attributed to hepatic VOD (two patients), septic shock (two patients), pulmonary edema (one patient), and infection (one patient). Four of the initial eight patients developed severe hepatotoxicity, and two of the patients with VOD had prior HSCT within 12 months. Therefore, the protocol was amended to exclude patients with prior HSCT, viral hepatitis and/or cirrhosis, or elevated conjugated bilirubin levels at study entry; there were no further events of severe liver toxicity in the remaining 17 patients. Nevertheless, among all 25 patients enrolled, the incidence of severe infections (grade 3 or greater) was high (72%). The study concluded that the combination treatment of clofarabine, etoposide, and cyclophosphamide in pediatric patients with refractory or relapsed ALL was effective in heavily treated patients but also suggested a need for optimization of the regimen with careful patient selection, dosing, and better supportive care to minimize toxicity.
The Children’s Oncology Group (COG) included this regimen in the post-induction consolidation phase for newly diagnosed, very high-risk patients with pre-B ALL [57]. The patients were randomized to a control arm (cyclophosphamide, fractionated cytarabine, mercaptopurine), experimental arm 1 (cyclophosphamide and etoposide), or experimental arm 2 (clofarabine 30 mg/m2 for 5 days, cyclophosphamide, and etoposide). All arms included vincristine and PEG-asparaginase, which were identical. More grade 4–5 infections and pancreatitis were seen in experimental arm 2 compared to the control arm or experimental arm 1. The dose of clofarabine was then reduced to 20 mg/m2 for 5 days and myeloid growth factor was required. However, 3 of 39 patients in experimental arm 2 with clofarabine developed grade 4 infections, compared to none in the control arm (n = 20) or experimental arm (n = 47). In addition, one of the patients who had a grade 4 infection developed grade 5 renal toxicity. Based on these results, experimental arm 2 was permanently closed.
Other studies using the same drug combination (clofarabine, cyclophosphamide, etoposide) [46, 58] had more favorable toxicity profiles but a similar efficacy. A study by Miano et al. investigated the same doses of the drugs used in the study described above (clofarabine 40 mg/m2/day, cyclophosphamide 440 mg/m2/day, and etoposide 100 mg/m2/day for 5 days). A total of 40 children with refractory or relapsed acute leukemia (24 ALL and 16 AML) were treated. Twenty of forty patients (50%) had prior HSCT. Seventeen patients (43%) had an overall response after the first cycle, which included nine CR (22%), eight CRp (15%), and two PR (5%). Seven of sixteen AML patients (44%) and ten of twenty-four ALL patients (42%) were responders. Twenty-six patients (65%) had at least one grade 3–4 toxicity, of which the most common toxicity was infection. Two patients who received the study treatment after HSCT died due to infection following prolonged bone marrow suppression after the first cycle. However, no death from liver toxicity was reported in this study. Locatelli et al. [46] used a slightly different dosing schedule: clofarabine 40 mg/m2/day, cyclophosphamide 400 mg/m2/day, and etoposide 150 mg/m2/day for 5 days (Table 17.4). Twenty-five children with refractory or multiple-relapsed ALL were enrolled in this study. Among them, eight patients were in a second (n = 6) or third (n = 2) relapse, and 17 patients were resistant to the last course of chemotherapy. Thirteen patients (52%) achieved CR and one (4%) achieved CRp; therefore, the ORR was 56%. Seven of the 13 patients (54%) who achieved a response proceeded to HSCT. The regimen was reported to be well tolerated. Febrile neutropenia, mucositis, and reversible liver toxicity were the most common AEs, but there were no cases of VOD, and most notably, no patients died of treatment-related complications. It should be noted that all patients received prophylactic antibiotics. Vigilant supportive care and dose modification may help to improve toxicity profiles with this regimen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree