Fig. 21.1
Numbers of patients recruited to US cancer trials by 5-year intervals (Personal communication with author)
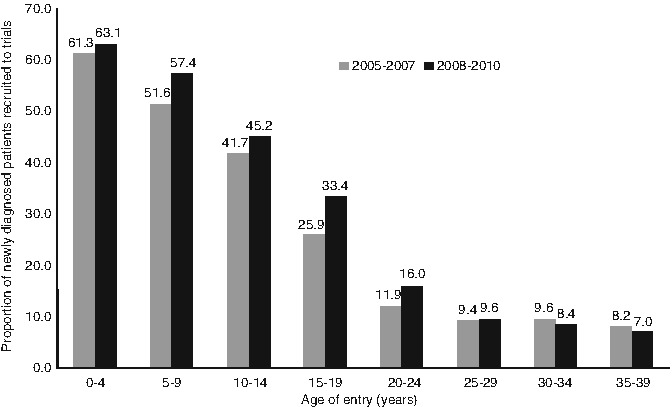
Fig. 21.2
Number of children, teenagers and young adults with newly diagnosed cancer entered onto selected clinical trials, 2005–2007 and 2008–2010, the United Kingdom
Access to early-phase trials or new agents can be an issue also for AYAs. Although this is described less fully, it is recognised generally that age is a barrier to accessing early-phase studies, particularly for adolescents [40]. Currently, in North America, the adolescent patient’s only access to new agents is through a paediatric phase 1 study. Since these studies are not initiated until the phase 2 dose for adults has been identified, there is a delay until the study is started, and then only a limited number of openings are available. Comparison of pharmacokinetic data between adults and adolescents suggests that there may be little difference between these groups, although these data should be evaluated on a case-by-case basis. In some cases, adolescent doses may be derived from the adult data and without the need for a dedicated pharmacokinetic study [41]. The current Children’s Oncology Group study AOST 1322 (Phase II Study of Eribulin in Recurrent or Refractory Osteosarcoma) is an example of this, since patients 12 years of age and older are eligible despite the fact that the paediatric phase I study of eribulin was initiated later.
The design of clinical trials continues to evolve to accommodate new forms of hypotheses and novel additions to the therapeutic armamentarium. There are particular challenges in accruing AYAs to cancer clinical trials, not the least of which is the paucity of high-quality data on the relevant issues.
21.2.3 Limitations to International Comparisons of AYA Accrual
It is recognised that recruitment of AYA to cancer clinical trials is an international problem in developed countries, as detailed in Sect. 21.2. However, the reporting of recruitment to these trials is in itself problematic, and therefore a comparison between countries is difficult. To date, no country has developed a comprehensive national reporting system for AYA accrual in terms of complete geographical coverage, all available studies (including industry studies) and a denominator that defines the number of eligible incident cases (the number of patients with a disease profile suitable for the available studies). Further, information on recruitment to earlier phase studies for relapsed/recurrent disease is largely unknown. While the methodology of data reporting varies between countries, the common trend of lesser involvement of AYAs compared to children and some older adults is consistent. This calls for a uniform mechanism to be identified to allow direct comparisons to be made, not only to make fair comparisons but to allow for evaluation of initiatives put in place to improve accrual.
21.2.4 The Case to Improve Accrual
Despite limitations of existing accrual data and a lack of empirical evidence of absolute gains to individual patients participating in clinical trials, improving AYA recruitment to cancer clinical trials seems a worthwhile pursuit. Given the evidence of improvements in survival for children with cancer, there is no doubt that there is much to be gained by incremental survival improvements observed over time related to trial activity [42]. In addition, collection of biological tissue along with outcome and toxicity data will serve to further our currently limited understanding of the molecular basis of cancers in AYAs [18].
In the healthcare systems of high-income countries, the inclusion of patients of all ages in well-designed phase 3 trials is considered the gold standard of care. Consequently, as AYA cancer care emerges as a distinct speciality, many of the initiatives that are being implemented have a focus on healthcare policy directives to increase participation of young people in cancer clinical trials. Countries such as the United Kingdom, the United States and Italy are now reporting improvements in recruitment of AYAs to cancer clinical trials [39, 43]. Despite these improvements, recruitment of children to such trials remains superior to that of AYAs and efforts to improve recruitment of AYAs are ongoing.
The generalisability of the benefits of new drugs or interventions is limited if not tested in the patient age range in which a disease is most likely to occur. As demonstrated in Fig. 21.3, less than 4 % of young adults with cancer in the United States were enrolled on national cooperative group trials between 2008 and 2010. Improving accrual would increase the generalisability of results, both in the AYA and older adult population. This phenomenon is well described for elderly patients, for trial participants tend to be younger than the mean age of patients with the disease and also free from co-morbidities common in the elderly population [44, 45]. In AYAs, the issues of co-morbidities are less; however, long-term toxic or late effects may not manifest in the remaining lifetime of an older population compared to young people who may survive for many decades beyond their treatment. In addition, the biology of disease in AYAs can be different from that in older adults and children [19], and therefore clinical response, particularly for new targeted therapies, may be altered or unpredictable. Issues related to fertility are also unlikely to be explored if the new agents are tested on a largely elderly population.
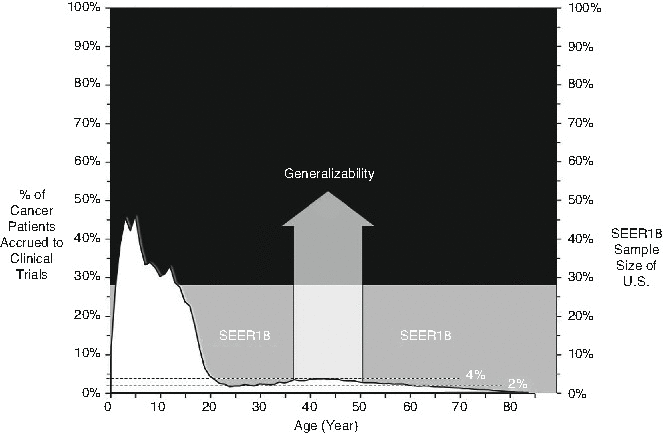

Fig. 21.3
Comparison of clinical trial accrual, registry data and the population for whom results are extrapolated. Estimated accrual to US National Cooperative Group clinical trials 2008–2010. SEER Surveillance, Epidemiology and End Results (Modified from JNCI 2015 courtesy of Archie Bleyer)
21.2.5 Challenges and Barriers to Accrual
There is no single reason for lesser involvement of AYAs in cancer clinical trials. The problem is multifaceted, and in an attempt to understand deficits in recruitment, researchers have turned historically to structural and organisational barriers for examination, for example, trial availability in centres where young people are treated and boundaries between paediatric and adult cancer care. More recently, the voice of the young person is being heard increasingly, and it is apparent that the reasons for lesser involvement of young people in cancer clinical trials may be more complicated than anticipated due to their unique psychosocial needs. The remainder of this section discusses some of the challenges and barriers to recruitment identified to date.
21.2.5.1 Appropriate Age Eligibility Criteria
Inclusion and exclusion criteria in clinical trials are important in defining a homogenous trial population. Medically and scientifically relevant parameters, such as disease and health status, restrict the study to those most likely to benefit safely from the intervention. However, the criteria that define the age of patients eligible for studies often have no medical or scientific rationale. Most commonly, age eligibility criteria reflect the source of the study, whether designed by paediatric or adult investigators, with adult studies typically having a lower age eligibility criterion of 18 years and paediatric studies having an upper age eligibility criterion ranging from 16 to 22 years. It is often these age eligibility criteria that deny young people access to research. Age eligibility criteria are applied to most studies despite statements in international healthcare documents that they should be avoided; see Box 21.1.
Box 21.1: International Statements Citing that Age Should Not Be Used as an Inclusion or Exclusion Criterion for Studies
UK Cancer Reform Strategy: ‘the use of age as an exclusion criterion in cancer clinical trials is avoided wherever possible’ [50].
Japanese Health Policy Bureau: ‘it is inappropriate to establish an arbitrary age limitation in clinical trial protocols’ [51].
Guidance for industry E11 clinical investigation of medicinal products in the pediatric population.
“The identification of which ages to study should be medicinal product – specific and justified” [52].
The Lancet Oncology: ‘especially problematic is the use of age as an exclusion criterion…widening patient eligibility criteria could lead to improved patient accrual, higher completion rates, and greater treatment equity’ [53].
In the United Kingdom, monitoring of studies following amendment of age eligibility criteria has shown that this influences accrual of AYAs positively, with increased numbers of AYAs enrolling onto trials following age eligibility amendment. Consequently, all new investigators submitting proposals to the major funder of research are being asked to provide scientific justification for their use of age eligibility, and if a lower limit must exist, it should be set at 16 years rather than 18 years. This serves two purposes, namely, that new studies in the United Kingdom will have lower age eligibility and also that investigators will begin to think more carefully about the use and relevance of any age-related restrictions for trial entry. A model of text that could be applied to other international funders of research, to allow greater access for AYAs, is shown in Box 21.2. The impact of restrictive age eligibility criteria on patients is clear in the case study of Chloe Drury in Box 21.3.
Box 21.2: Is Age to Be Included as an Inclusion/Exclusion Criterion?
If yes, please provide specific justification for both upper and lower age limits. Please note that if a lower age limit for studies involving adults is deemed essential, it should normally be set at 16 rather than 18 years. Low incidence of patients aged 16 and 17 is not sufficient reason for selecting a lower age criterion of 18 years.
Box 21.3: Case Study (Chloe)
The origins of age eligibility criteria are unclear, although the protection of paediatric patients is paramount and an obvious starting point. In the United Kingdom, the case of Chloe Drury, a 17-year-old patient unable to enter a new agent study until she was 18 and who later died from her disease, highlights the impact of exclusion by age for young people. Chloe was diagnosed with Ewing sarcoma in February 2010 aged 15 years, a true adolescent cancer with little improvement in outcomes over the past two decades. Three years later, having failed therapy, she was then denied access to a new agent being trialled at her local hospital. The study was open to patients aged 18 and over, typical for adult studies. Chloe was just short of her 18th birthday. Despite relentless campaigning by her mother, charitable bodies and healthcare professionals, Chloe had to wait until she turned 18 to enter the study. She died shortly afterwards.
That drug offered us a last tiny bit of hope. I remain incredulous that we were blocked from accessing that drug because Chloe was considered “too young”. She was 17 and nine months old. The lower age limit was 18. My daughter was denied a final shot at life because of a bureaucratic impediment. It is crazy and unbelievably cruel. We must do everything possible to stop this happening to another family. Please ask yourself what would you do if this was your child? Debbie Binner (Chloe’s mum)
The impact of restrictive age eligibility criteria is clear in Chloe’s case. Whether early access to the new agent would have prolonged Chloe’s life is unknown; however, her family is left with a sense of frustration and injustice that the new agent was withheld based solely on age rather than clinical risk or exhaustion of alternatives. It is difficult to explain particularly to grieving parents, why, in this era of personalised medicine and in a country that boasts the highest rate of clinical trial participation in the world, young people are being denied access to new agents simply because they are not old enough. Further, there is a fundamental lack of knowledge of the host and cancer biology in young people upon which we can base targeted drug development reliably; excluding young people from studies of new agents augments this problem further.
In the United Kingdom, the major funder of cancer research is now asking investigators to justify the use of age eligibility criteria on new funding applications, and if a lower age eligibility is needed, it should be set at 16 years rather than 18 years. A great step forwards…until the next Chloe is 6 weeks short of her 16th birthday. Access to studies should be based on disease and physiological status rather than age. However, there is no ‘quick win’ solution. Change will require new ways of thinking to ensure that basic scientists, clinical triallists, governments and regulatory and research agencies achieve greater equality in access to studies that in turn will offer more generalisable results.
21.2.5.2 Availability and Access
Whether a trial is available is a key determinant of recruitment. Cancers that effect young people are often rare and low in incidence compared to the more common types such as carcinomas of the breast, colon, lung and prostate. This can affect the availability of trials for young people at two levels. Low incident numbers, and therefore limited returns on investment, make trials of drugs for rarer cancers, such as those which occur in AYAs, relatively unattractive to the pharmaceutical industry, and trials are often not available for the most common cancer types occurring in young people.
The availability of trials in treatment centres is also a barrier to recruitment. In most countries, young people will be referred generally to an adult or paediatric institution, depending on their referring physician. Even in countries with well-established AYA care programmes, not all young people reach specialised AYA care; in the United Kingdom, this is approximately half of all young people. Treatment within a paediatric setting tends to result in higher rates of accrual of AYAs to trials compared to treatment in an adult setting. For example, a US study examining trial accrual of AYAs in affiliated paediatric and adult cancer centres found around a quarter (26 %) of all AYAs treated in the paediatric setting were enrolled onto trials compared to just 4 % of AYA, treated at adult centres [46]. This is related most likely to a greater availability of relevant trials and also to the ethos of recruitment to studies as the gold standard of care within paediatric oncology. Despite higher recruitment of AYAs to clinical trials in the paediatric setting, recruitment remains less than in children; a single-centre study, also in the United States, demonstrated that recruitment of patients less than 15 years of age was 38 % of all new patients compared to 27 % of new patients aged 15–22 years [30]. It is likely that lack of available trials is a contributing factor, as the spectrum of malignant disease changes with advancing age and trials available in children’s centres will reflect common children’s cancers that are different from those observed in adolescents and in particular young adults in whom carcinomas become more common [47]. In the aforementioned study, lack of trial availability was cited as a reason for non-recruitment in 41 % of paediatric cases, 57 % of those in the older age group [30]. For young adults with early-onset carcinomas, such as those of the breast or colon, the biology of their disease [19] may not be aligned with the availability of trials in adult centres where the majority of patients are older.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree