Fig. 11.1
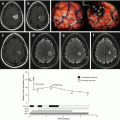
MRI of a patient with an oligodendroglioma WHO grade II in the right frontal pole. A right frontal lobectomy was performed with en-bloc removal of the tumor. Postoperative MRI confirmed that the tumor had been completely resected. An area with a high density of tumor cells was detected outside the radiological border. Left: An IDH1 image (with tumor cells brown-stained by the anti-IDH1-R132H antibody) showing the radiological border delineated by a solid line and the tumor cells outside the radiological border indicated by a dashed line. Right: The corresponding preoperative T2-FLAIR image with the radiological tumor border delineated by a solid line and the area with tumor cells missed on MRI delineated by a dashed line (from [4], J Neurosurg. 2016 Feb 26:1–12; reprints made with permission from the publisher)
More than 90% of all patients with DLGG are diagnosed during the symptomatic period and 70–90% of these patients have new-onset seizures as the first tumor-related symptom [6]. In general, seizure semiology reflects the specific location of the tumor with the somatotopic organization of cortical brain functions. Brain tumors give rise to partial (also called focal) seizures that occur with or without secondary generalized tonic-clonic seizures. The epileptic origin of partial seizures, in particular those originating from temporal or fronto-insular regions, may sometimes be difficult to recognize. Seizures may therefore go unnoticed for long time periods and it may not be until tonic-clonic seizures arise that patients seek medical ward.
Not all patients with DLGG present with new-onset seizures. DLGG are sometimes diagnosed incidentally in patients who undergo radiological examination for reasons or symptoms not related to the tumor [7]. This is in line with the natural course of DLGG that is traditionally considered to occur as a sequential three-step process; an initial silent period, followed by a symptomatic period, and a final period of malignant progression. From a clinical point of view, such a schematic view of the step-wise development of DLGG over time may be misleading. For example, in the silent phase with tumor diagnosis still unknown, insidious clinical symptoms may be present but unrecognized [8, 9]. Furthermore, malignant progression may occur prior to the development of neurological deterioration, indicating that the absence of progressive symptoms does not protect against malignant transformation [8].
Not surprisingly, the feeble association between tumor-related symptoms and the natural course of disease makes traditional clinical surveillance (“watchful waiting”) inadequate to detect important biological changes within DLGG. As the biological clock of DLGG may change suddenly to reveal a more aggressive biology, there is a fine line between what is considered “too early” and what may be “too late” with respect to tumor treatment. In other words, regardless of when patients are diagnosed within this continuum, there is a need to systematically consider early surgical intervention in light of the favorable impact of this strategy over “watchful waiting” for long-term outcome [10]. Interestingly, a recent trial showed that chemotherapy was also associated with better outcome when delivered early compared to as a rescue therapy at the time of tumor transformation [11]. These data illustrate the fundamental changes in view over the years in favor of early and maximal safe treatment for these differentiated and slowly proliferating tumors, which can be considered as a paradigm shift. They also underline the importance of a more comprehensive understanding of tumor-related symptoms and signs at presentation, and how these parameters relate to the molecular characteristics of the tumor, which is the focus of this chapter.
11.2 Clinical Symptoms
11.2.1 Incidental DLGG
It has been estimated that 3–10% of all DLGG are discovered incidentally, i.e. when radiological examination is performed for reasons unrelated to the tumor [12, 13]. In general, patients with incidental DLGG have smaller tumor volume, less frequent involvement of eloquent areas, and better performance score [14]. A recent study reported a high prevalence of subjective complaints but also of attention deficits and disturbances of working memory and executive function in patients with incidental DLGG, suggesting that these patients are to be considered as “not yet diagnosed” rather than “asymptomatic” [8]. It is not clear whether incidental DLGG constitute a specific subpopulation of tumors, and more studies are needed to clarify this important issue. In a retrospective case series of 23 incidental DLGG having surgical treatment, the majority consisted of IDH1-mutated, 1p/19q-codeleted oligodendrocytomas that in itself is a favorable prognostic subgroup [14].
11.2.2 Epileptic Seizures
Partial seizures due to brain tumors are divided in simple partial and complex partial seizures. There is no impairment of consciousness in case of simple partial seizures, while patients with complex partial seizures experience varying levels of clouded consciousness. Simple partial seizures are most common in patients with high-grade gliomas, complex partial seizures are more frequent in DLGG. In general, complex partial seizures consist of an initial phase that precedes clouding of consciousness (patients will be able to recall and report these initial experiences), followed by involuntary movements called automatisms such as lip smacking, chewing or picking at clothes. However, epileptic symptoms may vary from one patient to the other and the separate seizure components cannot always be distinguished. As mentioned, partial seizures may go unnoticed for long time periods and patients seek medical ward first when a secondary generalized tonic-clonic seizure arises.
Seizure semiology reflects the specific tumor localization with the somatotopic organization of cortical brain functions. The most common location of DLGG is the frontal lobe. Asymmetric tonic seizures, characterized by tonic arm extension and elevation followed by forced head deviation to the side of the extended arm (often but not always contralateral to the tumor) are strongly associated with frontal tumor locations, especially with seizure origin in the supplementary motor area [15]. Partial Jacksonian motor or somatosensory seizures, examples of simple partial seizures, are related to tumor location in perirolandic areas. Other typical manifestations of frontal lobe seizures are speech arrest or motor agitation, the latter characteristically occurring at night or upon awakening in early morning, and in patients with deeply seated frontal lobe tumors. This seizure type is sometimes mistaken for non-epileptic psychogenic seizures.
Temporal lobe seizures can be difficult to differentiate from frontal lobe seizures [16]. Patients with partial complex seizures due to temporal lobe tumors typically report characteristic symptoms consisting of déjà vu phenomena, visceral sensations such as epigastric rising, gustatory or olfactory auras. Auditory hallucinations, language or memory disturbances may also be part of temporal lobe seizures.
Seizures originating in the insular cortex may mimic temporal lobe seizures. In a series of 50 patients referred for preoperative evaluation of temporal lobe epilepsy, six patients with insular seizures had discharges on ictal EEG recordings that were distinct enough to allow differentiation from seizures with temporal origin [17]. In full consciousness these patients with pure insular seizures reported laryngeal discomfort with thoracic and abdominal oppression or dyspnea, unpleasant paresthesiae or warmth sensations in the face and extending to larger cutaneous territory, eventually followed by dysarthric speech and focal motor convulsions [17].
A less common location for DLGG is the occipital region. Patients with occipital lobe tumors may experience partial seizures consisting of positive visual symptoms such as flickering or blinking lights or visual disturbances such as micropsy and macropsy, but may also have visual field defects or blurred vision during seizure activity as in migraine.
11.2.3 Neurological and Cognitive Symptoms
The majority of patients with DLGG do not show sensorimotor deficits at presentation or during the entire pre-malignant phase of the disease. Standard neurological examination of patients with DLGG in eloquent location at the time of diagnosis is therefore usually normal. The slow growth of DLGG allows for cortical adaptor mechanisms, leaving time to reorganize the brain [18]. This brain plasticity that is characteristic for DLGG does not or only to a very limited extent, occur in patients with fast growing brain tumors such as glioblastomas. As a consequence, patients with high-grade gliomas frequently show neurological deficits at presentation [19]. The plasticity of subcortical structures is however limited, and patients with DLGG that infiltrate subcortical connectivity may also suffer from early functional deficits. The prevalence of functional deficits in patients with DLGG at clinical presentation is probably underestimated. Indeed, it has been estimated that more than 90% of all patients with brain tumors has at least some cognitive impairment prior to therapy [20].
Neuropsychological examination prior to any treatment is the only way to decipher tumor-induced cognitive changes from treatment-induced cognitive deficits. Further, the presence of cognitive deficits is strongly correlated with the health-related quality of life of patients with brain tumors [21, 22]. To our mind, this clearly justifies the inclusion of neuropsychological examination as part of the diagnostic procedure at disease presentation. Most of our knowledge on cognitive performance at the time of diagnosis comes from observational studies where cognitive function at diagnosis has been evaluated as baseline performance prior to treatment. Wu and co-workers compared neuropsychological function in patients with insular gliomas with a matched control group with non-insular gliomas and found frequent impairment in learning and memory in both groups [23]. Patients with insular tumors exhibited worse function in tests on naming [23].
In the elderly population of DLGG, sensorimotor deficits as well as cognitive impairment and language disorders at diagnosis are more often present while seizures occur less frequently as initial symptoms compared to younger patients [24]. These age-related differences in symptoms reflect a generally more aggressive tumor growth in the elderly population, favoring neurological and cognitive deficits over seizures in elderly patients with DLGG.
11.2.4 Mental-Health Related Symptoms
Depression and anxiety in patients with DLGG at clinical presentation may be caused by the psychological stress of brain tumor diagnosis, by the tumor itself or by a combination of these factors. Importantly, preoperative depression was associated with shorter survival in patients with DLGG [25]. Patients with tumor location in the right hemisphere had higher anxiety levels than patients with left sided primary brain tumors [26]. Still, brain tumor sidedness does not seem to have a large impact on the overall quality of life [27]. Behavioral disorders with changes in personality do sometimes occur in patients with DLGG but are more frequently found in patients with glioblastomas [22].
Fatigue as a multidimensional symptom including concentration problems, reduced motivation and physical activity, is frequently reported by cancer patients. Fatigue as a symptom related to DLGG has been studied mostly with regard to treatment or in long-term survivors [28]. In this population, fatigue was a severe problem in a large proportion of patients, with highest levels in elderly patients and patients using anti-epileptic drug treatment [28]. Six out of 15 patients with incidental DLGG reported subjective complaints of tiredness [8]. Thus, tiredness and other insidious symptoms may occur already during the silent phase of DLGG, demonstrating once more that patients with incidental DLGG are not necessarily asymptomatic. It may be argued that such unspecific symptoms are just as common in the normal population and thus not necessarily caused by the tumor. On the other hand, it was reported that 36% of patients with DLGG encountered adjustments in work tasks or reduced workload already one year prior to tumor diagnosis, which is higher than expected in the normal working population [29]. These data suggest that disease-related psychological and/or neurocognitive symptoms may precede radiological tumor diagnosis and underscore the importance of a comprehensive neurological and neuropsychological examination at initial visit to the clinic.
11.2.5 Implications of Symptoms for Clinical Management
Since many patients with DLGG present with seizures and show normal function at routine neurological examination, many centers have practiced “watchful waiting” as their primary strategy [10]. Even though this strategy does not jeopardize short-term quality of life, the cumulative quality of life of patients may be severely impaired due to earlier malignant transformation and death [30]. It is also worth noting that medically refractory seizures, which are amongst the most disabling symptoms, may respond well to extensive surgery [31]. Thus, extensive surgery is a potent symptomatic treatment for the most important tumor-related symptoms at presentation and provides long-term benefits in terms of improved survival.
Concerning the optimal treatment strategy for incidental DLGG, it is currently difficult to decipher the apparent excellent results from surgical treatment of incidental DLGG from the effects of the beneficial molecular profiles of these tumors and lead-time bias [7, 12, 14, 32]. It should always be born in mind that brain surgery itself may cause morbidity in terms of neurological impairment, cognitive disabilities and impaired quality of life. Nevertheless, since incidental DLGG demonstrate similar growth dynamics as symptomatic DLGG, early intervention is likely to be beneficial and to contribute to better chance of radiological complete tumor removal [10, 33]. Even though more studies are needed on both short-term and long-term implications of early tumor resection, the adverse effects of surgery seem to be acceptable for patients with incidental DLGG. Based on the current knowledge, most patients should therefore be given the option and advice to undergo early resection regardless of symptoms at presentation.
11.3 Radiological Presentation
11.3.1 Morphological MRI: Characteristic Findings
The RANO (Response Assessment in Neuro-Oncology) group for DLGG has provided criteria for the diagnosis and follow-up of non-enhancing tumors [34]. According to these criteria, morphological magnetic resonance imaging (MRI) is the imaging modality of choice for initial diagnosis with a protocol including non-enhanced and enhanced T1-weighted sequences, T2-weighted and T2-fluid attenuated inversion recovery (FLAIR) [34]. As such, MRI shows anatomical tumor location, tumor size, and contrast enhancement at presentation and is used to determine the individual tumor growth rate by repeated volumetric measurements over time. In general, DLGG show MRI signal intensities with hypointensity on T1-weighted images and hyperintensity on FLAIR or T2-weighted sequences (Fig. 11.2). The absence of contrast enhancement after administration of gadolinium-based MR contrast agent is characteristic for DLGG, but has low specificity and low sensitivity to differentiate DLGG from anaplastic gliomas. Approximately 20% of DLGG enhance, whereas approximately one third of non-enhancing gliomas consist of high-grade gliomas [35, 36]. Especially low-grade oligodendrogliomas may show minimal to moderate patchy, multifocal contrast enhancement [37]. This has been reported in up to 50%, distinguishing oligodendrogliomas from other DLGG, and is thought related to the tight capillary network that is a histological hallmark of these tumors [37].
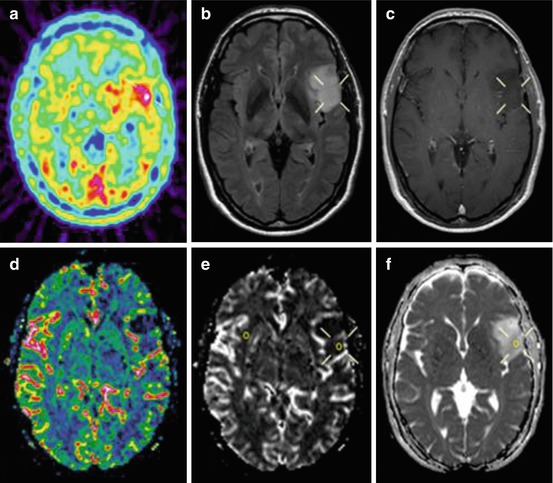

Fig. 11.2
Preoperative 11C-methionine-PET and MRI of a patient with a left-sided frontal astrocytoma grade II. (a) PET study shows the hot spot area in the tumor (hot spot/cortex ratio 1.6). (b) T2-weighted FLAIR MRI shows a high signal intensity tumor. (c) T1-weighted contrast-enhanced MRI shows a non-enhancing hypointense tumor. (d) Perfusion-MRI with rCBV color map shows low perfusion in the tumor area. (e) Perfusion-MRI with rCBV grey-scale map shows low perfusion in the region corresponding to hot spot on PET. The region of interests in the tumor and the contralateral normal appearing white matter are shown. (f) Diffusion-MRI shows increased mean diffusion in the region corresponding to the hot spot on PET
11.3.2 MRI-Based Estimation of Tumor Extension
Morphological MRI tends to underestimate the extension of DLGG [37]. Previous studies by multiple biopsies have identified tumor cells outside the radiological border on FLAIR or T2-weighted MRI sequences [38]. A recent report using co-registration of histology with MRI in en-bloc removed DLGG demonstrated tumor cells at 1–2 cm outside the radiological tumor borders, defined as the normal-appearing brain on FLAIR or T2-weighted images, in all tumors [3] (Fig. 11.1).
11.3.3 Volumetric Assessment of Initial Growth Dynamics
The assessment of volumetric growth may provide valuable information for differential diagnosis, especially when non-tumorous lesions or more uncommon variants of WHO grade II tumors like gangliogliomas are considered. If the tumor grows similar to the often quoted 4 mm/year in diameter, the likelihood increases that the image finding indeed is a DLGG [40]. Also, the initial growth rate may provide valuable prognostic information in DLGG [41, 42]. For widespread clinical implementation, more studies of measuring volumetric assessment including the inter- and intra-observer variability of the manual or semiautomatic methods are welcomed.
11.3.4 Advanced MRI
Advanced MRI methods like perfusion (pMRI) and diffusion (dMRI) imaging, providing information on tumor vascularity respectively cellularity, are now valuable diagnostic tools in neuro-oncology (Fig. 11.2). The pMRI parameter maximal cerebral blood volume (CBVmax) reflects neo-vascularization and correlates with malignancy grade [43, 44]. DLGG lack microvascular proliferation and have generally lower regional CBV (rCBV) values than high-grade gliomas [45]. In clinical practice, pMRI of DLGG is still limited to visual interpretation of perfusion maps representing mean values of measured parameters. Oligodendrogliomas tend to have higher rCBV values than astrocytic tumors, due to higher cell density and typical dense capillary networks in oligodendrogliomas.
dMRI measures the random motion of water molecules in tissues, reflecting their microarchitecture. Tumor diffusion as measured by dMRI provides quantitative information about tissue water diffusion and correlates with tumor cell density [45]. Diffusion is decreased in malignant gliomas due to a higher cellularity with restricted motion of water molecules in the extracellular space. In spite of generally lower mean diffusion (MD) in high-grade gliomas than in DLGG, it is difficult to predict tumor grade by dMRI due to the marked overlap in MD values between gliomas of different histological subtype and grade [46].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree