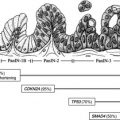
Pancreatic neuroendocrine tumors are a rare group of neoplasms that arise from multipotent stem cells in the pancreatic ductal epithelium. Although they comprise only 1% to 2% of pancreatic neoplasms, their incidence is increasing. Most pancreatic neuroendocrine tumors are nonfunctioning, but they can secrete various hormones resulting in unique clinical syndromes. Clinicians must be aware of the diverse manifestations of this disease, as the key step to management of these rare tumors is to first suspect the diagnosis.
Key points
- •
Pancreatic neuroendocrine tumors are a rare group of neoplasms, most of which are nonfunctioning.
- •
Functional pancreatic neoplasms secrete hormones that produce unique clinical syndromes.
- •
The key management of these rare tumors is to first suspect the diagnosis; to do this, clinicians must be familiar with their clinical syndromes.
Pancreatic neuroendocrine tumors (PNETs) are a rare group of neoplasms that arise from multipotent stem cells in the pancreatic ductal epithelium. Most PNETs are nonfunctioning, but they can secrete various hormones resulting in unique clinical syndromes. Clinicians must be aware of the diverse manifestations of this disease, as the key step to management of these rare tumors is to first suspect the diagnosis. In light of that, this article focuses on the clinical features of different PNETs. Surgical and medical management will not be discussed here, as they are addressed in other articles in this issue.
Epidemiology
Classification
PNETs are classified clinically as nonfunctional or functional, based on the properties of the hormones they secrete and their ability to produce a clinical syndrome. Nonfunctional PNETs (NF-PNETs) do not produce a clinical syndrome simply because they do not secrete hormones or because the hormones that are secreted do not cause specific symptoms. NF-PNETs are discovered incidentally on imaging or are detected as a result of symptoms related to tumor mass, invasion of adjacent structures, or metastatic disease. Functional PNETs (F-PNETs) are much less common and present with specific clinical syndromes related to their hormonal secretions. Diagnosis of F-PNETs is based on the presence of this clinical syndrome and diagnostic hormonal and functional studies; diagnosis is not based on immunocytochemistry. Both F-PNETs and NF-PNETs may secrete multiple peptides. Table 1 reviews the characteristics of the 9 commonly recognized PNETs. In addition to these 9, other rare PNETs have been described and new syndromes proposed but in not enough patients to result in a well-defined syndrome. The biologically active peptides secreted from rare F-PNETs in the literature include luteinizing hormone, erythropoietin, insulinlike growth factor II, enteroglucagon, renin, glucagon-like peptide–1, glucagon-like peptide–2, and pancreatic polypeptide. In addition, there are peptides known to be secreted from NF-PNETs with the theoretic potential to produce a clinical syndrome, although they have never been described as doing so. These peptides include calcitonin, neurotensin, pancreatic polypeptide, ghrelin, and subunits of human gonadotropin.
| Tumor (Syndrome) | Hormone | Clinical Presentation | Location | Malignancy |
|---|---|---|---|---|
| NF-PNETs | Eg, Pancreatic polypeptide, chorionic gonadotropin alpha, neuron-specific enolase | Related to tumor mass, invasion of adjacent structures, or metastatic disease | Pancreas 100% | 60%–90% |
| Insulinoma | Insulin | Whipple’s triad; neuroglycopenic, sympathetic | Pancreas 100% | <10% |
| Gastrinoma (Zollinger-Ellison) | Gastrin | Peptic ulcer disease, gastroesophageal reflux disease, diarrhea | Duodenum 70%, pancreas 25%, other 5% | 60%–90% |
| Glucagonoma | Glucagon | NME, DM, gastrointestinal symptoms, deep vein thrombosis, neurologic symptoms | Pancreas 100% | >60% |
| VIPoma (Verner-Morrison) | VIP | WHDA | Pancreas 90%, other 10% | 70%–90% |
| Somatostatinoma (SSoma) | Somatostatin | Tumor mass related, DM, gallbladder disease, weight loss, diarrhea, anemia | Pancreas 50%, duodenum and jejunum 50% | 60%–70% |
| GRFoma | Growth hormone–releasing factor | Acromegaly | Pancreas, lung, small intestine | 30%–50% |
| ACTHoma | ACTH | Cushing syndrome | Pancreas 100% | 95% |
| PTHrp-oma | PTHrp | Hypercalcemia | Pancreas 100% | >85% |
| PNET causing carcinoid syndrome | Serotonin, tachykinins | Carcinoid syndrome | Pancreas 100% | 60%–90% |
Pathology, Staging, and Grading
PNETs make up a heterogeneous group of tumors not only in their clinical features but also in their pathology. PNETs were previously referred to as islet cell tumors because of their resemblance to the islets of Langerhans. Although it was once thought that these tumors arose from neuroendocrine cells that migrated from the neural crest, it is now apparent that enteropancreatic neuroendocrine cells originate from multipotent stem cells that give rise to all epithelial cell types in the pancreas and gastrointestinal tract.
Lack of a uniform pathologic classification system has contributed to our lack of understanding of PNETs. Per recent consensus guidelines shared by the European and North American Neuroendocrine Tumor Societies and the World Health Organization, PNETs are now graded and staged separately.
In the 2010 World Health Organization classification system, PNETs are divided into 3 grades based on 2 factors: mitotic count and ki-67 labeling index. The system further divides PNETs into well-differentiated neuroendocrine tumors, made up of grade 1 and 2, and poorly differentiated neuroendocrine tumors, comprising grade 3. This finding reflects an increasing belief that poorly differentiated PNETs should be regarded as a completely separate entity from ordinary, well-differentiated PNETs.
There are several current staging systems for PNETs. The combined American Joint Commission on Cancer/Union of International Cancer Control/College of American Pathologists TNM staging system is similar to that used for exocrine tumors. It defines T3 tumors as those with peripancreatic spread. Unfortunately, most PNETs protrude from the pancreas even when small. The European Neuroendocrine Tumor Society TNM staging system relies more on tumor size for T stage.
Incidence
Pancreatic neuroendocrine tumors have an incidence of 1 to 5 per million per year. Autopsy studies suggest a greater frequency, occurring in 0.5% to 1.5% of the population. PNETs make up only 1% to 2% of pancreatic neoplasms; however, their incidence is increasing. NF-PNETs in particular may be increasing in incidence and are increasingly diagnosed in earlier stages of disease. This early diagnosis is likely because of increased incidental detection on imaging studies performed for another reason. The order of frequency of PNETs from most to least frequent is NF-PNETs, insulinoma, gastrinoma, glucagonoma, VIPoma, and others. Pancreatic neuroendocrine tumors are most frequently seen in adults but can rarely occur in children, and in these instances they are likely to have a hereditary predisposition. PNETs most often manifest in the fourth to sixth decades, occurring equally between the sexes, although poorly differentiated PNETs may have a higher incidence in men.
Etiology and Inherited Pancreatic Neuroendocrine Tumors Syndromes
Most PNETs are sporadic, but they are also seen in association with a few specific hereditary syndromes, including multiple endocrine neoplasia type 1 (MEN1), von Hippel–Lindau syndrome (VHL), neurofibromatosis type 1 (NF1), and tuberous sclerosis.
Almost all patients with MEN1 will have PNETs, most commonly microscopic and clinically insignificant NF-PNETs; however, F-PNETs also occur in MEN1. Of those with F-PNETS, 54% will have gastrinomas, 18% will have insulinomas, and less than 5% will have other types. These F-PNETs occur at an earlier age in patients with MEN1 than in sporadic cases and are more likely to be multiple.
PNETs develop in 10% to 17% of patients with VHL, almost always NF-PNETs. The mean age of PNET diagnosis in this population is 29 to 38 years, and 67% to 70% have a single PNET.
NF1, also known as Von Recklinghausen disease, has an uncommon incidence of PNETs, reported in up to 10%. These are almost exclusively duodenal somatostatinomas, usually occurring in the periampullary region. The behavior of duodenal somatostatinomas in patients with NF1 is similar to that of sporadic duodenal somatostatinomas in most respects.
Less than 1% of patients with tuberous sclerosis, also known as Bourneville disease, subsequently have PNETs. Both F-PNETs and NF-PNETs have been reported.
Epidemiology
Classification
PNETs are classified clinically as nonfunctional or functional, based on the properties of the hormones they secrete and their ability to produce a clinical syndrome. Nonfunctional PNETs (NF-PNETs) do not produce a clinical syndrome simply because they do not secrete hormones or because the hormones that are secreted do not cause specific symptoms. NF-PNETs are discovered incidentally on imaging or are detected as a result of symptoms related to tumor mass, invasion of adjacent structures, or metastatic disease. Functional PNETs (F-PNETs) are much less common and present with specific clinical syndromes related to their hormonal secretions. Diagnosis of F-PNETs is based on the presence of this clinical syndrome and diagnostic hormonal and functional studies; diagnosis is not based on immunocytochemistry. Both F-PNETs and NF-PNETs may secrete multiple peptides. Table 1 reviews the characteristics of the 9 commonly recognized PNETs. In addition to these 9, other rare PNETs have been described and new syndromes proposed but in not enough patients to result in a well-defined syndrome. The biologically active peptides secreted from rare F-PNETs in the literature include luteinizing hormone, erythropoietin, insulinlike growth factor II, enteroglucagon, renin, glucagon-like peptide–1, glucagon-like peptide–2, and pancreatic polypeptide. In addition, there are peptides known to be secreted from NF-PNETs with the theoretic potential to produce a clinical syndrome, although they have never been described as doing so. These peptides include calcitonin, neurotensin, pancreatic polypeptide, ghrelin, and subunits of human gonadotropin.
| Tumor (Syndrome) | Hormone | Clinical Presentation | Location | Malignancy |
|---|---|---|---|---|
| NF-PNETs | Eg, Pancreatic polypeptide, chorionic gonadotropin alpha, neuron-specific enolase | Related to tumor mass, invasion of adjacent structures, or metastatic disease | Pancreas 100% | 60%–90% |
| Insulinoma | Insulin | Whipple’s triad; neuroglycopenic, sympathetic | Pancreas 100% | <10% |
| Gastrinoma (Zollinger-Ellison) | Gastrin | Peptic ulcer disease, gastroesophageal reflux disease, diarrhea | Duodenum 70%, pancreas 25%, other 5% | 60%–90% |
| Glucagonoma | Glucagon | NME, DM, gastrointestinal symptoms, deep vein thrombosis, neurologic symptoms | Pancreas 100% | >60% |
| VIPoma (Verner-Morrison) | VIP | WHDA | Pancreas 90%, other 10% | 70%–90% |
| Somatostatinoma (SSoma) | Somatostatin | Tumor mass related, DM, gallbladder disease, weight loss, diarrhea, anemia | Pancreas 50%, duodenum and jejunum 50% | 60%–70% |
| GRFoma | Growth hormone–releasing factor | Acromegaly | Pancreas, lung, small intestine | 30%–50% |
| ACTHoma | ACTH | Cushing syndrome | Pancreas 100% | 95% |
| PTHrp-oma | PTHrp | Hypercalcemia | Pancreas 100% | >85% |
| PNET causing carcinoid syndrome | Serotonin, tachykinins | Carcinoid syndrome | Pancreas 100% | 60%–90% |
Pathology, Staging, and Grading
PNETs make up a heterogeneous group of tumors not only in their clinical features but also in their pathology. PNETs were previously referred to as islet cell tumors because of their resemblance to the islets of Langerhans. Although it was once thought that these tumors arose from neuroendocrine cells that migrated from the neural crest, it is now apparent that enteropancreatic neuroendocrine cells originate from multipotent stem cells that give rise to all epithelial cell types in the pancreas and gastrointestinal tract.
Lack of a uniform pathologic classification system has contributed to our lack of understanding of PNETs. Per recent consensus guidelines shared by the European and North American Neuroendocrine Tumor Societies and the World Health Organization, PNETs are now graded and staged separately.
In the 2010 World Health Organization classification system, PNETs are divided into 3 grades based on 2 factors: mitotic count and ki-67 labeling index. The system further divides PNETs into well-differentiated neuroendocrine tumors, made up of grade 1 and 2, and poorly differentiated neuroendocrine tumors, comprising grade 3. This finding reflects an increasing belief that poorly differentiated PNETs should be regarded as a completely separate entity from ordinary, well-differentiated PNETs.
There are several current staging systems for PNETs. The combined American Joint Commission on Cancer/Union of International Cancer Control/College of American Pathologists TNM staging system is similar to that used for exocrine tumors. It defines T3 tumors as those with peripancreatic spread. Unfortunately, most PNETs protrude from the pancreas even when small. The European Neuroendocrine Tumor Society TNM staging system relies more on tumor size for T stage.
Incidence
Pancreatic neuroendocrine tumors have an incidence of 1 to 5 per million per year. Autopsy studies suggest a greater frequency, occurring in 0.5% to 1.5% of the population. PNETs make up only 1% to 2% of pancreatic neoplasms; however, their incidence is increasing. NF-PNETs in particular may be increasing in incidence and are increasingly diagnosed in earlier stages of disease. This early diagnosis is likely because of increased incidental detection on imaging studies performed for another reason. The order of frequency of PNETs from most to least frequent is NF-PNETs, insulinoma, gastrinoma, glucagonoma, VIPoma, and others. Pancreatic neuroendocrine tumors are most frequently seen in adults but can rarely occur in children, and in these instances they are likely to have a hereditary predisposition. PNETs most often manifest in the fourth to sixth decades, occurring equally between the sexes, although poorly differentiated PNETs may have a higher incidence in men.
Etiology and Inherited Pancreatic Neuroendocrine Tumors Syndromes
Most PNETs are sporadic, but they are also seen in association with a few specific hereditary syndromes, including multiple endocrine neoplasia type 1 (MEN1), von Hippel–Lindau syndrome (VHL), neurofibromatosis type 1 (NF1), and tuberous sclerosis.
Almost all patients with MEN1 will have PNETs, most commonly microscopic and clinically insignificant NF-PNETs; however, F-PNETs also occur in MEN1. Of those with F-PNETS, 54% will have gastrinomas, 18% will have insulinomas, and less than 5% will have other types. These F-PNETs occur at an earlier age in patients with MEN1 than in sporadic cases and are more likely to be multiple.
PNETs develop in 10% to 17% of patients with VHL, almost always NF-PNETs. The mean age of PNET diagnosis in this population is 29 to 38 years, and 67% to 70% have a single PNET.
NF1, also known as Von Recklinghausen disease, has an uncommon incidence of PNETs, reported in up to 10%. These are almost exclusively duodenal somatostatinomas, usually occurring in the periampullary region. The behavior of duodenal somatostatinomas in patients with NF1 is similar to that of sporadic duodenal somatostatinomas in most respects.
Less than 1% of patients with tuberous sclerosis, also known as Bourneville disease, subsequently have PNETs. Both F-PNETs and NF-PNETs have been reported.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree