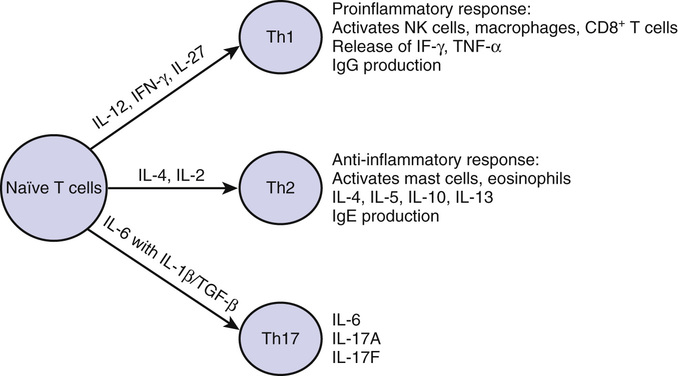
Mohan K. Tummala, Dennis D. Taub, William B. Ershler As a fundamental organ necessary for the maintenance of life, the immune system first appeared in primitive organisms about 480 million years ago.1 The intricate relationship between acquired immunity and infection was apparent early in recorded history. Observing an epidemic of plague in 430 BC, Thucydides reported that anyone who had recovered from the disease was spared during future outbreaks. The era of modern immunology was launched with Jenner’s report in 1798 of an effective vaccine using cowpox pustules to prevent smallpox in humans. Improved understanding of immunity and infection continued throughout the nineteenth and twentieth centuries. For example, identification of bacterial organisms ultimately resulted in the discovery of antibodies that could neutralize these microbes and/or their toxins, eventually leading to endorsement of the concept of vaccination. The discovery of antibody structure during the 1960s finally began the era of modern immunochemistry. With regard to cellular immunity, despite the early work of Metchnikoff and his followers, the role of cells in acquired immunity was not truly appreciated until the 1950s. Although theories of self-recognition and autoimmunity appeared early in the twentieth century, autoimmune diseases remain incompletely understood. As a concept, immunogerontology is a relatively recent focus of interest. In 1969, Walford proposed that declining immune function contributes to the biologic processes of aging.2 He speculated that disorders in the immune system that occur with aging account for three major causes of disease in older adults—increased autoimmunity, failing surveillance, allowing the expression of cancers, and increased susceptibility to infectious diseases. Current evidence supports the notion that the decline in immune function with aging may be viewed as a form of acquired immunodeficiency of modest dimension. Complicating the assessment of aging on immune function, older people are more likely to have diseases, conditions, or exposures that contribute to declining immune function.3 Primary (innate) immunity is the first line of defense against invading pathogens. It differs from secondary (acquired) immunity in that it does not require sensitization or prior exposure to offer protection. Primary immunity involves tissues (e.g., mucocutaneous barriers), cells (e.g., monocytes, neutrophils, natural killer [NK] cells) and soluble factors (e.g., cytokines, chemokines, complement) coordinated to mediate the nonspecific lysis of foreign cells. A feature of innate immunity is the detection of pathogens using pattern recognition receptors such as Toll-like receptors (TLRs), which recognize specific molecular patterns present on the surface of pathogens that trigger a variety of signaling pathways. After processing of antigen by the antigen-presenting cells (APCs), the peptide fragments are presented along with major histocompatibility (MHC) class II molecules to CD4+ T cells or with MHC class I molecules to CD8+ T cells to generate efficient T cell responses. The APCs also provide additional costimulatory stimulus (e.g., ligation of B7.1 or CD80 on APCs with CD28 on T cells) to lower the threshold of T cell activation and survival following the recognition of antigens. The ligation of TLRs on APCs enhances the phagocytosis of the pathogen through the release of chemokines and other peptides, which then result in the activation and recruitment of immune cells to the sites of infection. Phagocytosis involves the engulfment, lysis, and/or digestion of foreign substances. The capacity of neutrophils, macrophages, and monocytes for phagocytosis is determined by their number and ability to reach the relevant site, adhere to endothelial surfaces, respond to chemical signals (chemotaxis), and complete the process of phagocytosis.4 The study of alterations in phagocytosis with age must then involve examinations of each of these steps, which is inherently more difficult in human populations than in disease-free, inbred animals. Extrapolation of studies of senescent mice to humans suggests that age itself does not attenuate the response to bacterial capsular antigens in a well-vascularized area such as the lung.5,6 Niwa and colleagues have reported a deterioration in neutrophil chemotaxis and increase in serum lipid peroxidase levels in the nonsurviving cohort of a 7-year longitudinal study, suggesting a preterminal but not necessarily normal aging alteration in these factors.7 However, age-related effectiveness in chemotaxis may be reduced in less vascular tissues in vivo, such as in the skin, which also has a number of other changes that may impair the ability of cells in the vascular compartment to reach a site of infection.8 Although older adults preserve the number and overall phagocytic capacity, in vitro neutrophil functions (e.g., endothelial adherence, migration, granule secretory behavior such as superoxide production, nitric oxide, and apoptosis) appear to be reduced with age,9–11 and significantly fewer neutrophils arrive at the skin abrasion sites studied in older adults.12 How this translates to immune response and immune-mediated repair in infected or otherwise physiologically stressed older adults remains unknown. Although the expression of TLRs and granulocyte macrophage colony-stimulating factor (GM-CSF) receptors are not diminished, ligation of these receptors results in altered signal transduction. With aging, alterations in signal transduction of these receptors may be involved in the defective function of neutrophils, with decreased response to stimuli such as infection with gram-positive bacteria.13,14 These changes in older adults, unlike in younger persons, could be the result of changes in the recruitment of TLR4 into lipid rafts and no-raft fractions (the domains on plasma membrane that play an important role in cell signaling) with lipopolysaccharide (LPS) stimulation.15 Similarly, the activation through GM-CSF on the surface of these cells is also altered in older adults because of an age-related presence of a phosphatase in the lipid raft, blocking cell activation and contributing to a decreased response to GM-CSF in neutrophils from older adults.16 Macrophage activation also appears to change with age; this may be partially attributable to a reduced interferon-γ (IFN-γ) signal from T lymphocytes.17,18 A decrease in the number of macrophage precursors and macrophages is observed in bone marrow.19 Although it is not clear if there is an age-associated decrease of TLR on the surface of aged macrophages, defective production of cytokines has been observed after TLR stimulation, possibly due to altered signal transduction.20,21 With aging, there is diminished expression of MHC class II molecules in humans and mice, resulting in diminished antigen recognition and processing by these APCs.19,22 In addition, activated macrophages from humans and mice produce higher levels of prostaglandin E2, which may negatively influence antigen presentation.19 Fewer signals at the site of infection may be a consequence of reduced numbers of activated T cells locally due to the reduced antigen-processing capacity of macrophages. Fewer T cells and the defective expression of homing markers to attract T cells from peripheral blood into inflamed tissues23 suggests that increased susceptibility of old mic—for example, to tuberculosis—reflects an impaired capacity to focus mediator cells and the additional cytokine they may express at sites of infection (see more on T cell changes with age in later discussion). These observations may help explain why late-life tuberculosis or reactivation tuberculosis occurs and remains clinically important in geriatric populations. The change in function of antigen-presenting dendritic cells (DCs) with aging is less well defined. A decrease in the number and migration of Langerhans cells in skin has been described in older adults,24 but their function remains sufficient for antigen presentation.25 In contrast, DCs from older adults who are considered frail have been demonstrated to have reduced expression of costimulatory molecules, secrete less interleukin12 (IL-12), and stimulate a less robust T cell proliferative response when compared with those who are not frail.26 Cell lysis is mediated through a variety of pathways, including the complement system, NK cell, macrophage-monocyte, and neutrophil activity. Complement activity does not appear to decline significantly with age, and neutrophil function also appears intact. However, in longitudinal studies of nonhuman primates, NK cell activity does appear to be affected by age27 and acute stressors such as illness.28 The functioning status of NK cells is dependent on a balance of activating and inhibitory signals delivered to membrane receptors.29 A well-preserved NK cell activity is observed in healthy older adults,30 explaining, in part, a lower incidence of respiratory tract infections and higher antibody titers after influenza vaccination.31 However, older adults with chronic diseases and frailty are characterized by lower NK cell cytotoxicity and a greater predisposition to infection and other medical disorders.32,33 Although little is known about any changes in expression of activating and inhibitory receptors in older adults, NK activation and cytotoxic granule release remain intact.30,34 Secretion of IFN-γ after stimulation of purified NK cells with IL-2 shows an early decrease, which can be overcome with prolonged incubation.35 IL-12 or IL-2 can upregulate chemokine production, although to a lesser extent than that observed in younger subjects.36 These observations suggest that NK cells have an age-associated defect in their response to cytokines, with a subsequent detriment in their capacity to kill target cells and synthesize cytokines and chemokines. There are well-defined alterations in cellular and humoral immunity with advancing age. In the cellular immune system, most studies have shown no significant changes with human aging in the total number of peripheral blood cells, including total lymphocytes, monocytes, NK cells, or polymorphonuclear leukocytes.35,37–41 The appearance of lymphocytopenia is associated with mortality in older adults, but is not an age-related finding.42–44 Most studies have shown no changes in the percentages of B and T lymphocyte populations in the peripheral blood,45,46 although chronically ill older adults may, in particular, have a decline in total T cell numbers. Equivocal changes in the ratio of helper cells to suppressor cells (T4/T8) occur in normal aging.39,40,45,47,48 These findings are in contrast to human immunodeficiency virus (HIV)–induced acquired immunodeficiency syndrome (AIDS) associated with a decreased T4/T8 ratio. Finally, there is a specific age-related increase in memory cells, cells that express the CD45 surface marker.49–52 The function of lymphocytes is altered with aging. This may be a consequence of decreased thymic function, an important factor for age-related changes in thymus-dependent immunity and adaptive T cell immunity. Declines in serum thymic hormone levels precede the decline in thymic tissue. By the age of 60 years, few of the thymic peptides are measurable in human peripheral blood,53 and the thymus undergoes progressive reduction in size associated with the loss of thymic epithelial cells and a decrease in thymopoiesis. Thymic hormone replacement may improve immune function in older adults,54,55 but there are no current clinical indications in this regard. T cells may be considered naïve or memory on the basis of prior antigen exposure and, with advancing age, there has been noted a relative expansion of the memory T cell pool. The competency of adaptive immune function declines with age primarily because of a dramatic decline in the production of naïve lymphocytes due to a decline in thymic output and an increase in inert memory lymphocytes (see later discussion). Naïve CD4+ T cells isolated from older adults and animals display decreased in vitro responsiveness and altered profiles of cytokine secretion to mitogen stimulation, expand poorly, and give rise to fewer effector cells when compared with naïve CD4+ T cells isolated from younger hosts. Naïve CD4+ T cells from aged animals produce about half the IL-2 as younger cells on initial stimulation with APCs. Also, the helper function of naïve CD4+ T cells for antibody production is also decreased.56 However, newly generated CD4+ cells in aged mice respond well to antigens and are able to expand with adequate IL-2 production, with good cognate helper function. Thus, these age-related defects in naïve CD4+ T cells appear to be a result of the chronologic age of naïve CD4+ T cells rather than the chronologic age of the individual. These aged naïve CD4+ T cells proliferate less and produce less IL-2 in response to antigenic stimulation than naïve CD4+ T cells that have not undergone homeostatic divisions in the peripheral blood. The mechanism underlying homeostasis-associated dysfunction of naïve CD4 T cells is not known. In contrast to naïve cells, however, memory CD4+ T cells are long-lived, maintained by homeostatic cytokines, and are relatively competent with age. Isolated CD4+ T cells from healthy older adults and old mice are normal in regard to antigen proliferation in vitro.57 Memory CD4+ T cells generated from a young age respond well to antigens over time, whereas memory CD4+ T cells derived from older age respond poorly.58 Memory T cells generated from aged naïve T cells, on stimulation, survive and persist well, but are markedly defective in proliferation and cytokine secretion during recall responses, with impaired cognate help for humoral immunity. Healthy older adults can mount a CD4+ T cell response comparable to that observed in younger individuals when vaccinated with influenza vaccine, but they exhibit an impaired long-term CD4+ T cell immune response to the influenza vaccine.59 Vaccination with influenza results in increased IL-2 secretion in response to viral antigen in vitro.60,61 However, the number of influenza-specific cytotoxic T cells declines with age, with no increase after vaccination.62 Alteration in cell surface receptor expression (e.g., the loss of costimulatory receptor CD28 on the surface of CD8+ T cells) is one of the most prominent changes that occurs with aging. CD28−CD8+ T cells are absent in newborns but become the majority (80% to 90%) of circulating CD8+ T cells in older adults. Functionally, these CD28−CD8+ T cells are relatively inert and have a reduced proliferative response to TCR cross-linking, but maintain their capacity for cytotoxicity and are resistant to apoptosis.63 This loss of CD28 expression is associated with a gain of expression of stimulatory NK cell receptors in CD28−CD8+ memory T cells, enabling their effector function as a compensation for impaired proliferation.64 There is a reduction of naïve CD8+ T cells with some degree of oligoclonal expansion of CD8+ T cells with age observed in healthy older adults.65 This expansion may reflect a compensatory phenomenon to control a latent viral infection or fill available T cell space as a result of diminished output of naïve T cells from the thymus. When this clonal expansion reaches a critical level, the diversity of the T cell repertoire is reduced, and its ability to protect against new infections is compromised, as seen when older adults are exposed to new antigens. For example, the effect of host age was studied in the severe acute respiratory syndrome (SARS) outbreak, and it was discovered that the antigen recognition repertoire of T cells was approximately 108 in younger adults but only 106 in older adults.66 Notably, most of the SARS mortality was observed in infected persons older than 50 years. The accumulation of CD28−CD8+ T cells is also found in viral infections, such as cytomegalovirus (CMV), Epstein-Barr virus (EBV), and hepatitis C virus, so CD28−CD8+ T cells may be derived from CD28−CD8+ T cells after repeated antigenic stimulation.67 This clonal expansion of CD28−CD8+ T cells appears to be associated with increased infections and failed response to vaccines in older adults. As a result of the combination of thymic involution, repeated antigen exposure, and alteration in susceptibility to apoptosis (increased for CD4 and decreased for CD8), the thymic and lymphoid tissue in the aged host becomes populated with anergic (nonresponsive) memory CD28−CD8+ T cells, resulting in impaired cell-mediated immunity. The potential for far-reaching effects of the presence of senescent T cells is illustrated by the correlation between poor humoral response to vaccination in older adults and an increase in the proportion of CD8 T cells that lack expression of CD28.68,69 There is also a decline in delayed-type skin hypersensitivity (DTH),70–73 and the assessment of this has become a useful measure of cell-mediated immunity. Generally, a battery of skin test antigens (usually four to six antigens) is required to assess DTH adequately. The number of skin test positive reactions declines with age, from more than 80% in younger individuals to less than 20% in older adults.73 As with most functional measures in geriatric populations, there is remarkable heterogeneity. In one study,72 17.9% of subjects older than 66 years and living at home were anergic compared with 41% living in a nursing home but able to care for themselves and 60% who were functionally impaired and living in a nursing home. Although skin testing is a good indicator of cell-mediated immunologic health, it is heavily influenced by acute and chronic illnesses and the component of anergy because of aging is difficult to discern. Furthermore, concomitant in vitro testing has suggested that not all anergic patients have impaired in vitro responses,37,74 suggesting that some of the observed skin test anergy may be technical (i.e., due to difficulty in intradermal injection in the skin of older adults) or be caused by a deficit in antigen presentation, as described earlier. Thus, both in vivo cutaneous DTH assessment and in vitro lymphocyte testing may be necessary to identify more adequately those who are truly anergic and presumably immunodeficient. The relevance of this type of determination is apparent by the repeated demonstrations of an association between anergy and mortality.43,72,73,75–77 The issue of an age-associated decline in DTH has particular relevance for the testing of past or current tuberculosis exposure.78–82 Acknowledging the high incidence of anergy in older adult patients, care must be given to assess response to control antigens, such as Candida, mumps, or streptokinase-streptodornase (SKSD) before concluding that a negative tuberculin reaction indicates the absence of tuberculosis exposure. Furthermore, for healthy older adults, false-positive skin test results may be observed in those who have had repeated testing (the so-called booster effect).82 In the humoral immune system, there are no consistent changes in the number of peripheral blood B cells with age. The decline in antibody production following vaccination in older adults is the result of reduced antigen-specific B cell expansion and differentiation, leading to the production of low titers of antigen-specific immunoglobulin (IgG). Most studies have indicated a mild to moderate increase in total serum IgG and IgA levels, with no change in IgM levels.83,84 Declines in antibody titers to specific foreign antigens have been noted, including naturally occurring antibodies to the isoagglutinins85 and titers of antibody to foreign antigens such as microbial antigens.86–90 Both the primary91 and secondary immune responses to vaccination are impaired. Older patients tend to have lower peak titers of antibody and more rapid declines in titers after immunization,92,93 with the peak titer occurring slightly later than in younger people (2 to 6 weeks, rather than 2 to 3 weeks postvaccination).94 In contrast, serum autoantibodies may have organ specificity, such as antiparietal cell, antithyroglobulin, and antineuronal antibodies.46,95–101 With aging, there is a decreased generation of early progenitor B cells, resulting in a low output of new naïve B cells, with clonal expansion of antigen-experienced B cells. This results in a limited repertoire in immunoglobulin generation (through class switch) in B cells as observed in older adults and old mice,102 with limited antigen-specific B cell expansion and differentiation leading to the production of reduced titers of antigen-specific IgG. The antibodies produced by older B cells are commonly of low affinity due to reduced class switching and somatic recombination in the variable region of the immunoglobulin gene necessary for antibody production and diversity. The generation of memory B cells is highly dependent on germinal centers, the formation of which is known to decline with age. The formation of germinal centers is dependent to some extent on interactions of B cells with CD4+ T helper cells, and the age-related quantitative and qualitative changes in T and B cells may account, in large part, for the clinically observed diminished response to vaccines. For example, although 70% to 90% of individuals younger than 65 years are effectively protected after influenza vaccination, only 10% to 30% of frail older adults are protected.105 Organ-nonspecific autoantibodies, such as antibodies to DNA and rheumatoid factors, also increase with age. Circulating immune complexes may also increase with advancing age.95,106 The reason why autoantibodies increase with age is not known. Several explanations are possible, including alterations in immune regulation and an increase in stimulation of B cell clones because of recurrent or chronic infections or increased tissue degradation. There has been an increased awareness of alterations in the production and degradation of cytokines with age (Table 93-1). In vitro studies to assess functional aspects of lymphocytes after stimulation with mitogens have shown a decline in proliferative responses, possibly as a result of decreased T cell lymphokine production and regulation, particularly IL-2.44,48,107,108 Decreases in the percentage of IL-2 receptor–positive cells, IL-2 receptor density, and expression of IL-2 and IL-2 receptor–specific mRNA in older adults have been reported.48,109 IL-2 production in response to specific antigens also declines. There is a profound decline in the proliferative capacity of T lymphocytes to nonspecific mitogens.46,48,73,110 In addition, antigen-specific declines in the proliferative potential of T cells have been demonstrated.70,111 The number and affinity of mitogen receptors on T lymphocytes do not change with age.112 However, the number of T lymphocytes capable of dividing in response to mitogen exposure is reduced, and the activated T cells do not undergo as many divisions.80 Superimposed on the accumulation of a relatively inert naïve T cell fraction observed with advancing age, there appears also to be a shift in predominance of helper T cell responses from type 1 (Th1) to type 2 (Th2). Cells of the Th1 type produce IL-2, IFN-γ, and tumor necrosis factor-α (TNF-α) and predominantly mediate cell-mediated immune and inflammatory responses, whereas cells of the TH2 type produce IL-4, IL-5, IL-6, and IL-10, factors that enhance humoral immunity (Figure 93-1).56 Whereas the decline in IL-2 and IL-12 may contribute to the observed decline in cellular immune function, the increase in proinflammatory cytokines (particularly IL-6) may contribute to the metabolic changes associated with frailty. It has been proposed that a chronic exposure to such proinflammatory signals contributes to the phenotype of frailty.113 In fact, elevated IL-6 levels have been shown to correlate well with functional decline and mortality in a population of community-dwelling older adults.114 Thus the inflammation-related biomarkers are powerful predictors of frailty and mortality115,116 in older adults, a phenomenon termed inflammaging.49 In the steady state (i.e., in the absence of stress, trauma, infection, or disease), IL-6 is tightly controlled, and serum levels are typically measured in the very low picogram range. Among the regulators of IL-6 are sex steroids (estrogen, testosterone) and, at menopause, detectable IL-6 levels appear in the blood in apparently healthy older women. This inappropriate presence of a circulating proinflammatory molecule has garnered great interest among biogerontologists because it provides a rational explanation for many of the phenotypic features of frailty and levels associated with a number of age-associated disorders, including atherosclerosis, diabetes, Alzheimer disease, and osteoporosis.117–120 Waldorf and coworkers91 have speculated that autoimmunity plays an important role in the aging process. Cohen has alternatively proposed that autoimmunity may play an important physiologic role in the regenerative and reparative process that is ongoing during aging.121 Certain autoimmune diseases have their highest incidence in older adults, such as pernicious anemia, thyroiditis, bullous pemphigoid, rheumatoid arthritis, and temporal arteritis, suggesting that the age-related increase in autoantibodies may have clinical relevance,122–127 although this latter point remains unproven. Autoimmunity may also play a role in vascular disease in older adults.128 Giant T cell arteritis is a common disease in older adults124,129 and is associated with degenerative vascular disease. Immune mechanisms may cause atherosclerosis, a final common pathway of pathology secondary to a variety of vascular insults.130 A number of antivascular antibodies have been described in humans131–134 that are associated with diseases of the vasculature. Antiphospholipid antibodies are associated with a variety of pathologic states of the vasculature, including stroke and vascular dementia, temporal arteritis, and ischemic heart disease.135–138 However, the exact mechanism whereby antiphospholipid antibodies cause vascular injury remains unknown.139 The increased occurrence of antiphospholipid antibodies with age140–142 and the association of these autoantibodies with vascular disease may represent a predisposing immunologic factor for immune-mediated vascular disease in older adults. Autoantibodies to vascular heparan sulfate proteoglycan (vHSPG) may also be important in vascular injury in older adults133 because vHSPG play an important role in normal anticoagulation and cholesterol metabolism.143 Age is the single greatest risk for cancer.144 It has long been postulated that immune mechanisms play an important role in recognizing and destroying tumor cells; thus, an age-associated decline in immune function might be invoked to explain the increased rate of cancer in older adults. The problem with this hypothesis is that as rational as it sounds, it has been very difficult to prove (see later discussion). Furthermore, there are other explanations for the observed increased malignant disease in older adults, not the least of which is the estimated prolonged time (measured in decades for many epithelial tumors) that it takes to sustain the multiple genetic and epigenetic events required for malignant transformation and tumor growth to the point of clinical detection. An alternative explanation suggests that the host and host factors change over time, favoring progression and expression in later life. These two hypotheses to explain the increase in late-life malignancy have aptly been described as “seed versus soil.”145 From an immunologic and soil standpoint, there are two principal observations that relate to malignancies and age—deregulation of proliferation of cells directly controlled by the immune system and evidence of increased malignancies in later life that could be hypothetically restrained by nonsenescent immunity. These will be discussed sequentially. Proliferative disorders of the lymphocyte are common in older adults. Although bimodal in incidence, the peak in late-life lymphoma includes a disproportionate incidence of nodular B cell types.146 Both older adults and mice commonly exhibit a monoclonal gammopathy (paraprotein) in the last quartile of their life span.147–150 Monoclonal gammopathies increase with age and may occur in 79% of sera from subjects older than 95 years.151–153 Radl and colleagues151 have defined four categories of age-associated monoclonal gammopathy—myeloma or related disorders, benign B cell neoplasia, immunodeficiency, with T cell greater than B cell loss, and chronic antigenic stimulation. They speculated that the third category is the most common and that this is what occurs with immune senescence. It is possible that age-associated immune dysfunction is initially associated with markers of aberrant immune regulation, such as increased levels of paraproteinemia and/or autoantibodies, which may later contribute to the pathogenesis of lymphoma. Monoclonal gammopathies may cause morbidity, particularly renal disease, in the absence of overt multiple myeloma.154 In a minority of cases of monoclonal gammopathies, a malignant evolution may occur.154–156 Multiple myeloma also demonstrates an age-related increase in incidence.157 Although treatment is not generally indicated for monoclonal gammopathies,152 treatment of myeloma is often useful. Another common malignant transformation of the lymphocyte in older adults is chronic lymphocytic leukemia.158 Non-Hodgkin lymphoma also increases in incidence with age, whereas Hodgkin lymphoma has a bimodal distribution.159 Finally, a discussion of cancer development and aging would not be complete without considering the importance of the decline in immunity and associated failure of immune surveillance.160–163 It has long been proposed that the decline in immune function contributes to the increased incidence of malignancy. However, despite the appeal of such a hypothesis, scientific support has been limited, and the topic remains controversial.164 Proponents of an immune explanation point to experiments in which outbred strains of mice with heterogeneous immune function were followed for their life span.165,166 Those that demonstrated better function early in life, as determined by a limited panel of assays available at the time on a small sample of blood, were found to have fewer spontaneous malignancies and a longer life than those estimated to be less immunologically competent. Furthermore, it is difficult to deny that profoundly immunodeficient animals or humans are subject to a more frequent occurrence of malignant disease. Thus, it would stand to reason that others with less severe immunodeficiency would also be subject to malignancy, but perhaps less dramatically. However, the malignancies associated with profound immunodeficiency (e.g., with AIDS or after organ transplantation) are usually lymphomas, Kaposi sarcoma, or leukemia and are not the more common malignancies of geriatric populations (e.g., lung, breast, colon, prostate cancers). Accordingly, it is fair to say that the question of the influence of age-acquired immunodeficiency on the incidence of cancer in older adults is unresolved. There is much greater consensus on the importance of immune senescence in the clinical management of cancer, including the problems associated with infection and disease progression. An aging immune system is less capable of mounting an effective immune response after infectious challenge; thus, infection in older adults is associated with greater morbidity and mortality.167,168 Most notable in this regard are infections with influenza virus, pneumococcal pneumonia, and various urinary tract pathogens. However, older adults are also more susceptible to skin infections, gastroenteritis (including Clostridium difficile infection), tuberculosis, and herpes zoster (shingles). There is also an increase in hospital- and nursing home–acquired infections in older adults. These susceptibilities to infection are due to immune senescence and other changes more common among older adults, such as the following: reduced ciliary escalator efficiency and cough reflex predisposing to aspiration pneumonia; urinary and fecal incontinence predisposing to urinary tract and perineal skin infections; and immobility predisposing to pressure sores and wound infections. Infections in older adults frequently present atypically.74,144,169 Older adults may not have typical hard signs of infection, such as spiking fever, leukocytosis, prominent inflammatory infiltrates on chest x-rays, or rebound tenderness for those with an acute abdomen. Thus, a change in mental status or mild malaise might be the only clinical indication of urinary tract infection or even pneumonia. Lower baseline temperatures may require the need for monitoring the change in temperature, rather than the absolute temperature. This is particularly true in frail older adults, for whom infections caused by unusual organisms, recurrent infections with the same pathogen, or reactivation of quiescent diseases such as tuberculosis or herpes zoster virus can be counted on to present atypically and also to be resistant to standard therapy.
Clinical Immunology
Immune Senescence and the Acquired Immunodeficiency of Aging
Changes in the Human Immune System with Aging
Changes in the Human Immune System with Aging
Nonspecific Host Defense
Phagocytosis
Cell Lysis
Specific Host Defense
Qualitative Changes in Cell Function
Changes in T Cell Function
Changes in B Cell Function
Cytokine Dysregulation and Aging
Clinical Consequences of Immune Senescence
Autoimmunity
Immune Senescence
Immune Senescence and Cancer
Immune Senescence and Infections in Older Adults
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Clinical Immunology
93