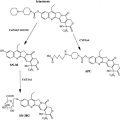
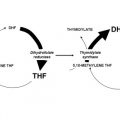
Advances are noted in the management of patients with highly responsive lymphomas to refractory solid tumors such as pancreatic cancer. Three articles illustrate how developments may take place even more than five decades from initial observations : mitotic inhibitors —long since Greenspan’s interest in podophyllotoxins and witnessing tumor lysis from leurocristine (later renamed vincristine) in two patients with hematologic malignancies; the antifolates aminopterin and later amethopterin (methotrexate) effect against acute lymphoblastic leukemia shortly after identification on folate pathways ; and topoisomerase-interacting drugs , joining 5-fluorouracil in showing benefit against gastrointestinal cancers—a decade before topoisomerases were identified. In fact, these old chemotherapies have proven to be quite “targeted.” A fourth article deals with PARP inhibitors with their effects on specific DNA repair pathways. Their role, alone or in combination, relies on selective enhancement of the consequence of DNA damage—often produced by a number of established anticancer drugs.
Kohler and Milstein were awarded the Nobel Prize in 1984 for hybridomas enabling production of monoclonal antibodies that gave rise to hopes for “magic bullets” against cancer. These molecules have moved slowly from diagnostics to a platform for therapeutic developments including diverse actions such as pathway inhibition, immune activation, and immunotoxins. Pioneering efforts of Judah Folkman on tumor angiogenesis resulted in the development of bevacizumab and has impacted on the treatment of several malignancies, but particularly on ovarian cancer. The role of anti-angiogenic including angiopoietin-targeted peptibodies is further covered in this issue. Monoclonal antibodies (such as cetuximab and panitumumab) and small molecules targeting the epidermal growth factor receptor as well as newly identified signal transduction pathways in lung cancer have changed systemic approaches to this most common of malignancies. The mTOR/PI3 kinase pathways activated via cell membrane receptor or by hypoxia, cellular metabolism, and proliferation provide new targets for treating Ewing’s sarcoma and renal cancer, among others. Inhibitors or the hedgehog pathway—responsible for key steps in stem cell biology and cellular remodeling—have already yielded remarkable clinical results in basal cell cancers and medulloblastoma. Finally, reprogramming of the genome by DNA methylation (by drugs such as azacytidine, initially developed as “cytotoxic” chemotherapy) is part of exciting new strategies against hematologic malignancies. As this issue attests, Louis Pasteur’s dictum “serendipity favors the prepared mind” certainly applies to clinical drug development 2012.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree