Antihypertensive class
Advantages
Disadvantages
Recommended indications
Cautions
Thiazide diuretics
Greater reduction of systolic BP
Daily use
Improve bone mineral density
Hypokalemia
Urinary frequency
Systolic hypertension
Hyponatremia
Gout
ACE inhibitors and angiotensin receptor blockers (ARB)
No CNS side effects
Preserve renal function
Reduce proteinuria
Cough
Hyperkalemia
HF
Type 2 Diabetes
Chronic kidney disease
Renal artery stenosis
Cough, altered taste, and angioneurotic edema with ACE inhibitors (try ARB)
Calcium channel antagonists (CCA)
No CNS side effects
No metabolic effects
Constipation
Peripheral edema
Heart block (Non-dihydropyridine CCAs)
HF with amlodipine
Systolic hypertension
CAD
Left ventricular dysfunction
Avoid short-acting CCAs for HTN
Beta adrenergic receptor blockers
None (not recommended as monotherapy)
CNS (Central Nervous System) side effects
Increased glucose and lipids with cardioselective
Post MI
CAD
HF
Atrial fibrillation with rapid rate (Beneficial in essential tremor, hyperthyroidism)
COPD (Chronic Obstructive Pulmonary Disease)
PAD
Heart block
Depression
Hyper-lipidemia
Type 2 DM
Alpha adrenergic receptor blockers
Improved urinary symptoms in Benign Prostatic Hypertrophy (BPH)
Increased CHF hospitalization
Prostatism
Left ventricular dysfunction
Renin inhibitors
Effective without dose-related adverse effects
No outcome data in the elderly
Expensive
Systolic hypertension
Diarrhea
Table 2
Reasons for poor c ontrol of blood pressure
• Inadequate dosing of antihypertensive drugs |
• Inappropriate drug combinations |
• Polypharmacy and increased incidence of adverse drug effects |
• Unrecognized contributing medical conditions or drugs |
• Practitioner inertia and failure to modify treatment |
Implications for Long-Term Care
The advantage of lowering blood pressure in the LTC population that are very old (>85 years), frail and have multiple competing comorbidities is not clearly established. At this time there are no data to support lowering of blood pressure to levels r ecommended by JNC 8 (less than 150/90 mmHg in nondiabetics, and less than 140/90 mmHg in those with diabetes) in persons over 80 years of age. Because the population in long-term care often has a limited life expectancy and is prone to developing orthostatic and post-prandial hypotension, syncope, and falls, the risks of lowering blood pressure with medication are increased and its potential benefit reduced. Data is lacking to help determine how best to treat elevated BP in residents with high-risk conditions such as recent stroke, functional impairment, and aortic aneurysm.
Anemia
Aging predisposes persons to decreased hematopoietic reserve, reduced absorption of essential nutrients, decline in GFR and erythropoietin secretion (EPO), increased concentrations of cytokines; but anemia is not considered a normal part of aging. Ane mia may have an insidious onset with nonspecific symptoms until it is becomes more severe. Mild anemia is often assumed as being benign or attributed to the presence of chronic comorbidities. However, studies in community dwelling elders show that anemia may be an independent risk factor for adverse outcomes when DM, CKD, or cardiovascular disease is present. Anemia has been associated with significant clinical problems and adverse outcomes (Table 3).
Table 3
Clinical imp act of anemia
Frailty |
Falls |
Fatigue |
Dizziness/syncope |
Shortness of breath |
Decreased muscle strength |
Cognitive impairment |
Impaired mobility and physical performance |
Heart failure |
Increased hospital admissions and mortality |
Definition and Prevalence of Anemia
There is no unifor m definition of anemia. The World Health Organization defines anemia as a hemoglobin (Hb) level less than 12 g/dL in adult women and less than 13 g/dL in adult men. These cutoffs are based on population data that did not include people > 65 years of age, and do not take into account the effect of race and ethnicity. From the NHANES III and the Scripps-Kaiser database, new lower limits of normal for men >59 years and women >49 years have been proposed after excluding those with confounding factors. These values were slightly higher for older white men and women (13.2 and 12.2 g/dL, respectively) and slightly lower for older black men and women (12.7 and 11.5, respectively). However, such levels may not be optimal with regard to morbidity and mortality. For example, in the Women’s Health and Aging Study, a risk gradient for adverse outcomes (mortality, frailty, disability) was present with Hb in the “normal range” as was a rise in the erythropoietin level [8]. In a multi-facility study, 56 % of residents were anemic; prior estimates have ranged from 25 to 63 % [9].
Signs and Symptoms
Signs and symptoms in long-term c are residents may be nonspecific (Table 4) so staff needs to be aware of their potential significance and report them to the practitioner.
Table 4
Nonspecific signs and sym ptoms of anemia
Anorexia, nausea |
Bleeding gums |
Chest pain, palpitations, tachycardia |
Cold intolerance |
Dizziness |
Decreased activity level or endurance |
Dyspnea |
Fatigue |
Increase in falls |
Increased confusion, headache |
Jaundice |
Melena |
Hematuria |
Pallor (skin, conjunctivae) |
Causes of Anemia
Anemia is ge nerally due to an underlying clinical disorder and warrants an evaluation unless the resident has a reduced life expectancy, is receiving palliative care, or declines further evaluation. A systematic evaluation can help the practitioner make rational treatment decisions. Using empiric iron replacement, for example, can potentially overlook a significant underlying treatable disorder. The causes of anemia may be classified by etiology, bearing in mind that more than one cause may be present in a given person.
Nutrient deficiency anemia (iron (IDA), folate, B12).
Blood loss (i.e., gastritis, AV malformation, diverticulosis, bladder tumor)
Anemia of CKD.
Anemia of chronic inflammation (ACI).
Medications (may cause bleeding or marrow suppression).
Myelodysplastic anemia (affects 5 % of older people).
Unclassified (may be observed in one-third of LTC residents).
However, an alternative approach to aide clinical decision-making can be more useful in most clinical settings. It includes assessing the resident’s medical history, comorbidities, renal function, current and recent medication use, physical findings, and review of laboratory tests. Anemia can then be classified by considering kinetics (decreased production, increased destruction, or blood loss), or by considering red cell morphology. The following algorithm (Fig. 1) suggests a diagnostic approach using the corrected reticulocyte count and MCV (as low, normal, or increased) prior to obtaining further tests. Table 5 lists suggested noninvasive diagnostic test for evaluation of anemia [10].
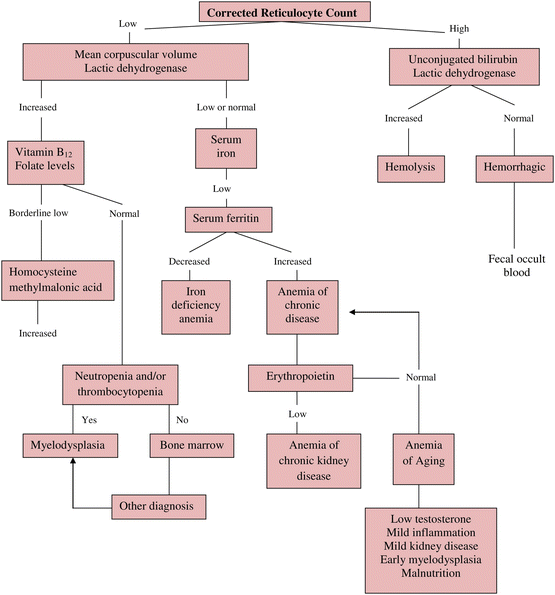

Fig. 1
Algorithm for the d iagnosis of anemia (Source: Morley JE. J Am Med Dir Assoc. 13(3):191–4)
Table 5
Noninvasive diag nostic tests
• Complete blood count with reticulocyte count (initial low Hb/Hct may not be evident in volume depletion) |
• Examination of peripheral blood smear |
• Ferritin, serum iron, total iron-binding capacity (serum soluble transferring receptor) |
• Serum folate |
• Vitamin B12 (methylmalonic acid, homocysteine) |
• Renal function (eGFR) |
• Liver function |
• Sedimentation rate |
• Tests for hemolysis (serum LDH, bilirubin, and haptoglobin) |
• Serum protein electrophoresis |
• Stool for occult blood (endoscopy if appropriate |
• Thyroid-stimulating hormone (free T4) |
• Total testosterone |
Sometimes it is difficult to differentiate iron deficiency anemia from anemia of chronic inflammation since the typical hematologic abnormalities of advanced iron deficiency occur at a later stage and both types of anemia can coexist. Table 6 will assist in analyzing equivocal results [11]. Measurement of the soluble transferrin receptor in conjunction with iron studies and ferritin, is gaining some acceptance in differentiating iron deficiency anemia from anemia of chronic inflammation.
Table 6
Lab value di fferentiation of iron-deficiency anemia from anemia of chronic inflammation
Blood test | ACI | IDA | ACI + IDA |
|---|---|---|---|
Iron | ↓ | ↓ | ↓ |
TIBC | ↓ | ↑ | LN or ↓↑ |
% Transferrin saturation | ↓ or N | ↓ | ↓ |
Ferritin | ↑ or N | ↓ | ↓ or N |
Soluble transferrin receptor | N | ↑ | ↑ or N |
Anemia of CKD
As renal f unction decl ines in people with CKD, the Hb will progressively decline. This drop in Hb is especially noticeable as the GFR trends below 60 mL/min per 1.73 m2. The anemia of CKD is typically normochromic and normocytic due primarily to a deficiency of erythropoietin production. About 50 % of nursing facility residents have a GFR below 60 mL/min per 1.73 m2 and in one recent study, 60 % of residents with Stage III CKD were anemic.
Acute or Chronic Immune Activation (ACI)
Acute or chro nic immune activation can cause a disturbance of iron homeostasis that limits the av ailability of iron for erythropoiesis due to the impaired release of iron from macrophages. This disturbance in iron homeostasis is mediated by the proinflammatory cytokine interleukin-6, which increases the production of hepcidin in the liver. Hepcidin decreases duodenal absorption of iron. In addition, erythropoietic cell survival and erythropoietic cell response to EPO is decreased.
Treatment
Treatment of como rbid nutritional deficiencies and hypothyroidism should be undertaken. If anemia is related to medication use, chronic bleeding, CKD, chronic inflammation, malignancy, or hemolysis, then the underlying condition should be stabilized to the extent possible and any offending drugs discontinued. Treatment options for specific types of anemia and cautions to consider are reviewed in Table 7.
Table 7
Treatment opti ons for anemia based on etiology
Cause of anemia | Treatment options | Cautions |
|---|---|---|
Iron deficiency | • Ferrous sulfate 325 mg daily (65 mg elemental iron) • Ferrous gluconate 300 mg daily (36 mg elemental iron) • Parenteral iron (in cancer, CKD) | • Constipation • GI distress • Consider blood loss |
Vitamin B12 deficiency | • Vitamin B12 1,000 μg IM weekly × 1 month, then monthly if neurological complications • Oral B12 500–1000 μg daily • Sublingual 2000 μg daily | • Check for concurrent folate deficiency |
Folate deficiency | • Folate l mg orally, daily for 2–3 weeks, then reevaluate the need for continued therapy | • Check for concurrent B12 deficiency |
Anemia of chronic inflammation | • Treat or stabilize the underlying disease | • Anemia may persist • Erythropoietin use is not approved |
Anemia of chronic kidney disease | • Epoetin alfa or darbepoetin alfa SC • Control diabetes and HTN | • Maintain Hb 10–11 g/dL • Weekly Hb till stable then monthly • Monitor BP |
Hemolytic anemia | • Identify underlying cause • Discontinue any contributing medications |
Blood transfusions are generally given for acute blood loss associated with hypotension and cardiovascular compromise. For chronic anemia, blood transfusion is recommended if the Hb drops below 7 g/dL, the hematocrit decreases to 21 %, or in the presence of angina, heart failure, dyspnea, tachycardia, or hypotension.
Heart Failure
Cardiovascular disease is the primary diagnosis for 25 % of admissions to nursing facilities. A study sampling of 10 % of skilled nursing facilities (SNFs) during 2003–2004 revealed that 37.4 % of patients had HF. Heart failure is responsible for signifi cant morbidity and readmissions to the hospital. The lifetime risk of HF doubles for BP >160/90 mmHg. Hypertension is a major risk factor for the development of HF, especially HF with preserved ejection fraction (EF) in long-term care residents. In one study HF with preserved ejection fraction (HFpEF) was present in 50 % of nursing facility residents diagnosed with HF. Heart failure with reduced EF of <45 % is termed HFrEF. Moreover, HF incidence increases significantly in older adults with diabetes and obesity, especially in females. Elderly with HF with either a markedly high or low BP have a worse prognosis as do those with an abnormal LVEF [12]. SNF rehospitalization rates for HF range from 27 to 43 %. Overall, mortality in LTC residents with HF exceeds 45 %, and hospitalization exceeds 50 % annually, although there may be regional differences [13].
Evaluation
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines for the evaluation and management of HF h ave classified this condition in four stages (A-D) [14]. The first two stages (A, B) are not symptomatic stages of heart failure but defined to help practitioners identify those at risk for developing HF. The NYHA classification can be used to clarify symptom severity (Table 8).
Table 8
New York Heart Association classification
Class | Patient symptoms |
|---|---|
Class I | No limitation of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation, or dyspnea (shortness of breath) |
Class II (mild) | Slight limitation of physical activity. Comfortable at rest, but ordinary physical activity results in fatigue, palpitation, or dyspnea |
Class III (moderate) | Marked limitation of physical activity. Comfortable at rest, but less than ordinary activity causes fatigue, palpitation, or dyspnea |
Class IV (severe) | Unable to carry out any physical activity without discomfort. Symptoms of cardiac insufficiency at rest. If any physical activity is undertaken, discomfort is increased |
The clinical differentiation between diastolic and systolic dysfunction although challenging, may be helpful in decision-making. A co mplete history and physical examination should be performed in residents with shortness of breath, reduced exercise tolerance, edema, or other symptoms suggestive of HF. Review of prior records may provide vital information regarding cardiovascular conditions. Current medications, use of alcohol, and/or illicit drugs and alternative therapies, as well as chemotherapy agents should also be considered as contributing factors to HF.
The manifestations of heart failure may be atypical in long–term care residents. Frail elderly may have fatigue, malaise, lethargy, declining function, or neurological symptoms such as confusion, restlessness, or sleep disturbance when they develop an exacerbation of HF. Orthopnea, dyspnea with exertion, cough or edema may be present. Gastrointestinal manifestations of HF may include anorexia, nausea, and abdominal discomfort.
Patients who h ave HFpEF (i.e., preserved EF) are more often female, have a fourth heart sound, sustained PMI, absence of jugular venous distension, absence of peripheral edema, normal heart size on chest -ray, and left ventricular hypertrophy (LVH) on the electrocardiogram (EKG). By contrast, patients with HFrEF (i.e., reduced EF) are more often male, have a third heart sound, displaced PMI, jugular venous distension, pitting edema, and Q waves on the EKG.
Clinical Diagnosis of HF
A physical exami nation is central to the diagnosis of HF and should include the weight (It is important to remember that edema found on physical exam may also be due to non-cardiac causes).
Electrolytes and renal function (to detect hyponatremia, potassium abnormalities, worsening of renal function, hypomagnesemia).
A chest X-ray is useful to estimate cardiac size, assess pulmonary congestion, and to detect other pulmonary disease.
While useful, a comprehensive 2-dimensional echocardiogram with Doppler flow studies (to assess LVEF, the presence of valvular, or pericardial abnormalities) may not be easily available.
An EKG may indicate the presence of Q waves, LVH, arrhythmias, or conduction disorders.
Screening for comorbid thyroid disease is also recommended (see section on hypothyroidism).
Serum assays for natriuretic peptides (BNP and NT-proBNP) are readily available and may lend weight to the suspicion of HF in residents in whom the cause of dyspnea is not clear. However, its use and applicability is unclear in the LTC setting [14].
Electrolytes and renal fu nction should be measured regularly as hypokalemia is a common adverse effect of diuretics and may increase the risk of fatal arrhythmias or digoxin toxicity. Many residents with hypokalemia also have hypomagnesemia, which can result in an inadequate response to potassium supplementation. Hyperkalemia can be associated with ACE inhibitors, angiotensin II receptor blockers, or worsening renal function. The development of hyponatremia may be an indication of disease progression and is associated with reduced survival in the elderly with HF.
Serial chest X-rays are not recommended but monitoring weights 2–3 times a week during an exacerbation of HF is useful practice. The resident’s functional status should be monitored in addition to the physical examination with sitting and standing BP.
Common Precipitants of Heart Failure
In addition to attempting to identify the cause of heart failure, it is also important to be aware of conditions that may precipitate an exacerbation of heart failure in order that the medication regimen and the treatment of these coexisting medical conditions can be optimized. Cardiologist consultation may be required in some instances (Table 9).
Table 9
Common factors precipitating HF
Cardiac |
Myocardial infarction or ischemia |
Poorly controlled hypertension |
Excess of dietary sodium |
Medication nonadherence |
Excess fluid intake (oral or IV) |
Arrhythmias—supraventricular (especially atrial fibrillation with rapid rate), bradycardia, sick sinus syndrome |
Associated medical conditions—pulmonary embolism, hypoxia due to chronic lung disease, infection (pneumonia, viral illness, sepsis), anemia, hyperthyroidism, chronic kidney disease (eGFR <30 mL/min) |
Medications—alcohol, -β adrenergic blockers (including ophthalmic agents), calcium channel-blockers, NSAIDS, glucocorticosteroids, mineralocorticoids, antiarrhythmic drugs |
Provider/system problems (e.g., medication reconciliation errors) |
Process of Care Considerations
Close observation, a nd early detection of symptoms and signs may precede an acute HF episode by several days. Timely intervention by the practitioner (with evaluation of weights, chest X-ray, electrolytes and renal function, adjustment of therapy) and regular monitoring by nursing staff may prevent hospitalization. Records of prior cardiac investigations and echocardiograms will assist in better defining the type of HF in terms of LVEF, LVH and valvular dysfunction. A study of 156 episodes of HF in 4693 Medicare nursing facility admissions within the first 90 days of stay, reported that symptom presentation and evaluation by nursing staff at night increased the odds of rehospitalization fourfold. The presence of hypotension and delirium were predictive of death. Residents who received ACE inhibitors and orders for skilled nursing observations more than once a shift decreased the likelihood of dying by 70 % [15]. Residents with anemia (Hb <9.8 g/dL) were twofold more likely, and those with CKD (stage III or greater) were fivefold more likely to be rehospitalized from a nursing facility HF rehabilitation unit.
Management
The followi ng reviews consensus recommendations for the management of HF and should be applied to each resident in an individualized manner [14]. The choice of pharmacologic and non-pharmacologic therapy will depend on the patient’s clinical status, goals of care, as well burden of comorbid conditions and prognosis. The suggested treatment paradigm can be based on three broadly identified groups in many facilities: the Rehabilitation Group in whom the goal is improved function and discharge; those who are frail with multi-morbidities and have an Uncertain Prognosis; and the Long–term Care Group who are frail, dependent for care needs, and expected to remain in the facility. Each recommendation should be individualized (Table 10).
Table 10
Suggested medical man agement of heart failure based on care goals
Intervention | Rehabilitation group | Uncertain prognosis group | Long-term group |
|---|---|---|---|
Assessment of LVEF | Yes | Yes | Preferable (individualize) |
Sodium restriction | Preferable | Preferable | Preferable, but individualize |
Diuretics to treat volume overload | Yes | Yes | Yes |
ACEIs/ARBs | Yes for HFrEF (avoid low SBP) | Yes for HFrEF (avoid low SBP) | Yes for HFrEF (avoid low SBP) |
β-blocker | Yes for HFrEF as tolerated (BP, HR, fatigue) | Yes for HFrEF as tolerated (BP, HR, fatigue) | Yes for HFrEF as tolerated (BP, HR, fatigue) |
Mineralocorticoid receptor antagonist | Yes for HFrEF NYHA II–IV and in NYHA III after MI; avoid in those with eGFR <30 mL/min | Yes for HFrEF NYHA II–IV and in NYHA III after MI; avoid in those with eGFR <30 mL/min | Yes for HFrEF NYHA II–IV and in NYHA III after MI; avoid in those with eGFR <30 mL/min |
Hydralazine-nitrates | Yes for HFrEF in black patients with standard therapy; If contraindications to ACEIs or ARBs or as adjunctive therapy for advanced HF | Yes for HFrEF in black patients with standard therapy; If contraindications to ACEIs or ARBs or as adjunctive therapy for advanced HF | Yes for HFrEF in black patients with standard therapy; If contraindications to ACEIs or ARBs or as adjunctive therapy for advanced HF |
Digoxin | Yes for HFrEF, only if symptomatic despite treatment with an ACEI or ARB, a β-blocker, and a mineralocorticoid receptor antagonist; low dose (≤0.125 mg/d) | Yes for HFrEF, only if symptomatic despite treatment with an ACEI or ARB, a β-blocker, and a mineralocorticoid receptor antagonist; low dose (≤0.125 mg/d) | Yes for HFrEF, only if symptomatic despite treatment with an ACEI or ARB, a β-blocker, and a mineralocorticoid receptor antagonist; low dose (≤0.125 mg/d) |
Implantable cardioverter defibrillator | Stable optimized medications for 3 months, LVEF ≤35 %, NYHA II–III, and expected survival of at least 12 months | Observe until recovery seems likely | Not indicated |
Cardiac resynchronization therapy | Persistent symptoms, optimized medications for 3 months, LBBB and LVEF ≤35 % and QRS ≥150 ms and NYHA II–IV | Observe until recovery seems likely | Not indicated |
Discuss and identify end-of-life preferences | Yes | Yes | Yes |
Cautions
Enc ourage moderate activity and physical therapy if possible.
Thiazides are ineffective if GFR is <30 ml/min/1.73 m2; use loop diuretics.
If symptoms persist and ACEI or ARB cannot be given due to decrease in GFR or hypotension, give isosorbide plus hydralazine, especially in African Americans.
Avoid calcium channel blockers.
Digoxin
Many re sidents may receive digoxin (sometimes for years) as a standard part of the treatment regimen for HF despite the presence of sinus rhythm and normal LVEF. The evidence indicates that low dose digoxin (digoxin concentration <1.0 ng/mL) may decrease mortality in HFrEF and NYHA II and III symptoms in elderly who do not have an adequate response to ACE inhibitors, beta-blockers, and diuretics.
The elderly are at increased risk of digoxin toxicity due to renal insufficiency, hypoalbuminemia, hypokalemia with hypomagnesemia, as well as drug–drug interactions. Drug interactions between digoxin and antiarrythmics, erythromycin and tetracycline can be especially problematic. Close monitoring, especially during acute illness causing dehydration, is recommended and serum concentrations should be maintained between 0.5 and 0.8 ng/ml [16].
Refractory HF
HF can ha ve an unpredictable course and a high incidence of sudden death despite intensive medical management. Palliative care should be considered for those with refractory HF and persistence of severe and distressful symptoms such as dyspnea, fatigue, pain, sleep disturbance, and functional decline.
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is an insidious, progressive lung disease characterized by airflow obstruction that is not f ully reversible. COPD may be difficult to diagnose because persons often gradually modify their lifestyle to compensate for progressive symptoms and dyspnea. COPD may be difficult to differentiate from asthma, HF, or other comorbidities that limit physical activities. COPD is the third leading cause of death in the USA. One in six patients admitted to a nursing facility may have COPD or emphysema, and it is responsible for 1 % of deaths in LTC [17].
Identification of COPD
Smoking (90 % of cases), advan ced age, repeated pulmonary infections, prior tuberculosis, occupational or environmental causes, and alpha-1 antitrypsin deficiency are risk factors for COPD. Early identification of COPD is important since 50 % of lung function is lost by the time mild exertional dyspnea occurs and only 30 % of lung function remains when there is dyspnea at rest. All new admissions to LTC and those residents with recurrent pulmonary problems should be screened for COPD utilizing the clinical indicators listed in Table 11 [18].
Table 11
Clinical indicators of COPD in long-term care
Dyspnea (progressive, worse with exertion) |
Cough (may be intermittent and unproductive) |
Chronic sputum production (any pattern) |
Avoidance of activities that lead to dyspnea |
History of smoking |
Recurrent pulmonary infections |
Occupational or environmental exposure to dust particles |
Weight loss, anxiety, or sleep disorders |
Diagnosis of COPD
On exami nation, residents may be barrel-chested, have prolonged expiration or pursed lip breathing, use accessory muscles for respiration, and have wheezing, ronchi, or distant heart sounds. The signs of cor pulmonale include jugular venous distension, hepatic congestion, pedal edema, and a loud P2 component of the second heart sound.
The clinical evaluation and review of past records may be helpful in diagnosing COPD, but the definitive method of diagnosis is by spirometry which usually measures FEV1 (volume of air exhaled in 1 s) to FVC (forced vital capacity or total volume of air able to be exhaled). Spirometry should be performed in symptomatic individuals and may need to be repeated.
Normal: FEV 1 /FVC ≥ 70 % or FEV 1 ≥ 80 % of predicted
COPD: FEV 1 /FVC ≤ 70 %
Restrictive lung disease: FEV 1 /FVC ≥ 90 % (pulmonary fibrosis, severe kyphosis)
COPD is und iagnosed in 80 % of older adults and not all LTC facilities have bedside spirometers and their use in frail or demented residents is usually not feasible. A screening tool that uses caregivers to rate residents’ symptoms was validated in the NF by Zarrowitz et al. They report that a history of asthma, shortness of breath at rest and shortness of breath on exertion, and smoking are likely to be consistent with a diagnosis of COPD.
Other diagn ostic tests may provide useful information regarding the presence of COPD or other conditions presenting with similar signs and symptoms. A complete blood count may reveal an abnormally high Hb level due to hypoxia, and a chemistry panel may show a high bicarbonate level (respiratory alkalosis) due to hypercapnea. Even though chest X-rays are not diagnostic, they may reveal HF, bullae, pneumonia, pulmonary scarring; or low flat diaphragms, increased retrosternal airspace and a teardrop shaped heart silhouette are suggestive of COPD. An EKG may show atrial arrhythmias or right heart strain. Pulmonary consultation may be helpful if the cause of dyspnea is not clear or the resident exhibits a poor response to treatment. Clinical judgment is important since the differential diagnosis of COPD can include asthma, heart failure, brochiectasis, recurrent aspiration, ACE-inhibitor induced cough, vocal cord dysfunction, and respiratory tract tumors. The GOLD criteria of COPD severity are shown in Table 12 [19].
Table 12
GOLD spirometry cri teria for COPD severity
I: Mild COPD |
• FEV1/FVC <70 % |
• FEV1 ≥80 % predicted |
II: Moderate COPD |
• FEV1/FVC <70 % |
• 50 % ≤FEV1 <79 % predicted |
III: Severe COPD |
• FEV1/FVC <70 % |
• 30 % ≤ FEV1 < 39 % predicted |
IV: Very severe COPD |
• FEV1/FVC <70 % |
• FEV1 <30 % predicted or FEV1 <50 % predicted plus |
Chronic respiratory failurea |
Management of COPD
Since staging of COPD by spirometry criteria is not usually possible or practical in PA/LTC, clinical presentation and judgment must be utilized to guide treatment [20] (Table 13).
Table 13
Management of COPD
Spirometry | Begin pharmacotherapy if symptomatic | ||
|---|---|---|---|
Pharmacotherapy | • Short-acting bronchodilators PRN • Short-acting bronchodilators (regular and PRN) • Long-acting bronchodilator and PRN short-acting beta-2-agonist • Long-acting bronchodilator + inhaled corticosteroida and PRN short-acting beta-2-agonist • Combination long-acting agents (long-acting beta-2- agonist + long-acting anticholinergic) + inhaled corticosteroids + as needed short-acting beta-2-agonist | ||
Severe progressive disease | Supplemental oxygen | Noninvasive positive pressure ventilation (patient is conscious, cooperative and without large volumes of sputum) | Theophylline |
Other interventions | • Smoking cessation • Influenza and pneumococcal vaccination • Osteopenia/osteoporosis evaluation • Pulmonary rehabilitation • Evaluate for lung-volume reduction surgery | ||
Plan of care | • End-of-life care directives • Reinforce proper inhaler technique • Depression and anxiety screening | ||
Encour aging smoking cessation is important at any stage of this disease; as are measures to improve nutrition, encourage physical activity and immunization with influenza and pneumococcal vaccines. Complications such as polycythemia, hypoxia, and HF should be treated, and goals of care should be discussed with the resident and family.
Pharmacological treatment should be stepwise and cumulative. Medications can reduce symptoms, increase exercise capacity, and reduce the number and severity of exacerbations; but no treatment has been shown to modify the progressive decline in lung function. Three types of bronchodilators are in common clinical use: β-agonists, anticholinergic drugs and inhaled corticosteroids.
Long-acting bronchodilators are more effective than short-acting bronchodilators or anticholinergics.
Anticholinergics given four times a day can improve health status.
A combination of short acting agents (salbutamol/ipratropium) produces a greater change in lung function than either agent alone.
Bronchodilators from different classes may improve efficacy, understanding that treatment needs to be long-term.
An inhaled corticosteroid combined with a long-acting beta-2 agonist is more effective than either agent alone and may reduce the frequency of exacerbations, as well as improve health status.
The inhaled route of treatment (i.e., use of MDIs) is preferred (Table 14).
Table 14
Commonly used pharmacologic agents: benefits and cautions
Drug class
Drug example
Dosage
Cautions
Short-acting β agonists
Albuterol MDI
1–2 inhalations every 4–6 h
All four drugs may be used for acute bronchospasm
Levalbuterol MDI
2 inhalations every 4–6 h PRN
Albuterol 2.5 mg
for nebulization
3 ml TID-QID
Levalbuterol 0.6 mg
3 ml TID
Long-acting β agonists
Formoterol DPI
1 inhalation (12 mcg) every 12 h
Not for acute bronchospasm. Palpitations, tremor, bronchospasm
Salmeterol DPI
1 inhalation (50 mcg) every 12 h
Arformotero
Nebulization (15 mcg) BID
Anticholinergics
Ipratropium bromide MDI
2–3 (17 mcg each) inhalations QID
May be used for acute exacerbation
Ipratropium bromide 500 mcg for nebulization
2.5 ml TID-QID
May be used for acute exacerbation
Tiotropium DPI
1 inhalation (18 mcg) daily
For maintenance treatment. Not for acute bronchospasm
Caution with BPH and glaucoma
Umecldinium
1 inhalation (62.5 mcg) daily
Aclidinium
One inhalation (400 mcg) BID
Glucocorticoids
Inhaled corticosteroids (MDI or DPI)
Beclomethasone diproprionate 40 mcg/inhalation
1–2 inhalations BID
For severe COPD with repeated exacerbations; added to routine bronchodilator therapy
Fluticasone DPI
2 inhalations BID
Oral corticosteroids
Prednisone 5 mg
30–40 mg/d for 10 days
Monitor glucose in patients with DM
Osteoporosis, myopathy and cataracts
Prednisolone 4 mg
24–32 mg/day for 10 days
Methylxanthines
Theophylline ER
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access