The current treatment paradigm for metastatic renal cell carcinoma (RCC) includes agents that target the vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR) pathways. Because these agents have revolutionized RCC over the past five years, new clinical and molecular predictive and prognostic tools are required. These are potentially important for therapy selection, patient counseling, and clinical trial stratification. This review examines clinical prognostic models and molecular biomarkers in RCC.
Before the advent of molecular targeted therapy, treatment of metastatic renal cell carcinoma (mRCC) involved immunotherapy with high-dose interleukin (IL)-2 in selected patients, interferon-α, and cytoreductive nephrectomy. Current standard therapies focus on inhibiting angiogenesis via the vascular endothelial growth factor (VEGF) pathway (sorafenib, sunitinib, pazopanib, and bevacizumab) or the mammalian target of rapamycin (mTOR) pathway (temsirolimus and everolimus). The treatment landscape of mRCC is rapidly evolving, thus predictive and prognostic factors must be continuously evaluated to reflect advances in systemic therapy.
A prognostic factor provides information about the patient’s overall disease outcome independent of any specific intervention. A predictive factor provides information about the probability of benefit or toxicity from a specific intervention. Many clinical prognostic factors remain unchanged; however, a myriad of biomarkers surrounding angiogenesis is emerging. Although most require prospective validation, they offer promising insights into diagnosis, predicting response to therapy, and prognostication of overall survival. These biomarkers are key elements that allow clinicians to potentially individualize cancer therapy, compare and risk-stratify patients in clinical trials, and accurately counsel patients on the status of their disease. This review discusses important findings in the realm of predictive and prognostic factors that are both clinical and laboratory based.
Clinical prognostic factors
Prognostic factors have been derived from patients treated in clinical trials as well as retrospective population-based databases, with survival as the primary end point. There are 4 general groups of prognostic factors: those associated with patient status, tumor burden, proinflammatory markers, and treatment-related factors ( Table 1 ). Many of these have been combined in multivariable analysis to enable discrimination between those who have a favorable, intermediate, and poor prognosis ( Tables 2 and 3 ).
| Category | Prognostic Factor |
|---|---|
| Patient-related factors | Symptoms |
| Performance status | |
| Tumor burden | Site and or number of metastatic sites |
| Alkaline phosphatase Hyponatremia | |
| Lactate dehydrogenase Anemia Hypercalcemia | |
| Disease-free interval | |
| Proinflammatory markers | Erythrocyte sedimentation rate C reactive protein Neutrophilia Thrombocytosis |
| Treatment | Cytoreductive nephrectomy |
| Model | MSKCC Motzer et al | UISS Zisman et al | Groupe Français d’Immunotherapie | Heng et al | Patil et al | Choueiri et al |
| Patient population | 463 patients treated with interferon-α on prospective clinical trials | Prospective cohort of 814 patients who underwent nephrectomy (Stage I–IV patients) | 782 patients treated with immunotherapy on trials | 645 patients treated with sunitinib, sorafenib, or bevacizumab at multiple North American centers | 375 patients treated with sunitinib from an RCT | 120 patients treated with bevacizumab, sorafenib, sunitinib, or axitinib on prospective clinical trials at a single center |
| Common prognostic factors compared with Motzer criteria | KPS <80% LDH >1.5× ULN Corrected calcium >10 mg/dL (2.5 mmol/L) Hemoglobin < LLN Disease-free interval <1 y | ECOG PS | ECOG PS Hemoglobin < LLN Disease-free-interval <1 y | KPS <80% Corrected calcium > ULN Hemoglobin < LLN Disease-free interval <1 y | ECOG status LDH > ULN Corrected calcium > ULN Hemoglobin < LLN Disease-free-interval <1 y | ECOG status Corrected calcium <8.5 mg/dL or >10 mg/dL Disease-free-interval <2 y |
| Significant prognostic factors specific to model | Fuhrman histologic grade 1997 TNM staging | Number of metastatic sites SR ≥100 or CRP ≥50 | Neutrophils > ULN Platelets > ULN | Bone metastases | Neutrophils >4500/μL Platelets >300,000/μL |
| Biomarker | Method | Role in Renal Cell Cancer | Comments |
|---|---|---|---|
| VHL alterations | Mutations: PCR, SSCP, sequencing Promoter hypermethylation: methylation-specific PCR | Conflicting evidence regarding role as prognostic factor Conflicting evidence regarding role as a predictive of response to targeted therapies | Larger studies focusing on VHL mutations with functional significance required to clarify prognostic and predictive role |
| HIF-1α | IHC | Conflicting evidence regarding role as prognostic factor Not predictive of response to interferon-α or temsirolimus | Nuclear and cytoplasmic staining should be assessed |
| Western blot | Predictive: high levels of HIF-1α or HIF-2α associated with increased response rate to sunitinib (n = 43) | Larger studies needed | |
| VEGF-A | ELISA | Potentially prognostic: high VEGF associated with poor OS Baseline levels not predictive of response to sunitinib, sorafenib, pazopanib, or bevacizumab plus interferon | Further work is required to define cut point between high and low VEGF and significant changes in VEGF |
| SNP | Predictive: VEGFA −1154 AA genotype (vs GG) associated with poor OS for patients treated with pazopanib (n = 241) but not for sunitinib (n = 63) | Confirmatory prospective trials needed | |
| CAIX | IHC | Prognostic role unclear in patients who had nephrectomy | Cutpoint of ≤85% for low versus >85% for high, established using survival tree analysis Caveat: CAIX expression in metastatic lesions may not be representative of primary tumors |
| Predictive role not established for targeted therapy | Larger studies needed | ||
| mTOR pathway | IHC | PTEN not predictive, prognostic role unclear PS6 expression predictive of response to temsirolimus in one small study | pAkt difficult to measure from paraffin-embedded tissue |
| B7-H1 | IHC | Prognostic: positive expression associated with shorter survival in patients who had nephrectomy for clear cell RCC | Predictive studies needed, especially in patients receiving PD-1 inhibitors |
| STAT3 | SNP | Polymorphism in 5′ region of STAT3, rs4796793, predictive of response to interferon-α in one small study | Larger studies needed in different ethnic populations |
| NGAL | N/A | High NGAL levels associated with worse PFS for patients on sunitinib (unclear if prognostic or predictive) | NGAL threshold of 110 ng/mL needs to be validated in larger studies |
| IMP3 | IHC | Prognostic: positive expression associated with shorter survival | Predictive studies needed |
| Thymidylate synthetase expression | mRNA | Predictive: low expression associated with improved PFS in cytokine refractory Japanese mRCC patients treated with S-1 (n = 45) | Should be evaluated as a predictive marker in future trials of S-1 |
Patient Factors
In a review of mRCC patients treated with IL-2–based immunotherapy after nephrectomy, survival was negatively affected by the presence of constitutional symptoms such as weight loss, decreased appetite, musculoskeletal pain, sweats, rashes, and respiratory and gastrointestinal symptoms. Poor performance status, as assessed by Eastern Cooperative Oncology Group (ECOG) or Karnofsky Performance Status (KPS) scales, is consistently associated with shortened overall survival for patients after nephrectomy, as well as in the metastatic setting.
Tumor Burden
Patients with greater than one site of metastases, no prior nephrectomy, and bone metastases have an associated poorer prognosis. Other markers of increased tumor burden are also associated with worse overall survival. These markers include elevated lactate dehydrogenase (LDH) due to high cell turnover, anemia, hypercalcemia due to bone metastases or paraneoplastic syndromes, and hyponatremia due to syndrome of inappropriate antidiuretic hormone secretion (eg, due to brain or pulmonary metastases) or paraneoplastic syndromes. The interval between diagnosis and the development or treatment of metastatic disease, also known as the disease-free interval (DFI), is inversely linked to prognosis, as it is an indirect measure of indolent versus aggressive disease.
Proinflammatory Markers
Markers of inflammation such as elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) have been linked to decreased overall survival in retrospective and prospective series. ESR and CRP have yet to be evaluated as prognostic factors in patients treated with targeted therapy. Neutrophilia is a poor prognostic factor for patients treated with antiangiogenic drugs in two multivariable prognostic-factor models but not in immunotherapy models. Although thrombocytosis is rare in patients with mRCC, its presence is associated with worse outcomes in some series. Thrombocytosis may be related to production of interleukin-6 and other growth factors by the tumor. In addition, platelet granules contain proangiogenic factors, which may promote tumor growth.
Treatment-Related Factors
For patients who present with metastatic disease and good performance status, performing cytoreductive nephrectomy prior to interferon-α therapy improves survival compared with interferon-α alone in two randomized studies.
Multivariable Prognostic Models
In light of the plethora of prognostic factors, multivariable models have been created to classify patients into poor, intermediate, and favorable risk groups for use in the clinic as well as in clinical trials (see Table 2 ). For Stage I to IV patients, the University of California Los Angeles integrated staging system (UISS) is the most widely studied prognostic model that correlates with postnephrectomy outcomes. The Mayo Clinic metastases-free survival score (SSIGN) was derived from analysis of 1671 patients who had a nephrectomy for clear-cell RCC (ccRCC), and subsequently was externally validated. Multivariate analysis revealed development of metastases was significantly associated with tumor stage, regional lymph node status, tumor size, nuclear grade, and histologic tumor necrosis ( P <.001 for all).
In the era of immunotherapy, the most well known and used prognostic model for mRCC was developed at the Memorial Sloan-Kettering Cancer Center (MSKCC) by Motzer and colleagues (see Table 2 ). This model stratified patients based on the number of risk factors: favorable risk (no risk factors), intermediate risk (1 risk factor), and poor risk (2–3 risk factors), with median overall survival times of 22.1 months, 12 months, and 5 months, respectively. The Groupe Français d’Immunotherapie derived a slightly different model based on 782 patients treated with immunotherapy on clinical trials (see Table 2 ). The MSKCC prognostic criteria were externally validated and expanded at the Cleveland Clinic ; thus it became the standard prognostic-factor model for patients with metastatic disease.
Additional prognostic-factor models have been created, based on mRCC patients treated with angiogenesis inhibitors and internally validated (see Table 2 ). These models are similar to the original MSKCC criteria. Two of the models did not observe an association between overall survival and LDH, and both these models found that neutrophilia and thrombocytosis were prognostic. The Heng criteria were derived from a large population-based database that segregated patients into 3 risk categories: favorable risk (no risk factors, median overall survival not reached), intermediate risk (1–2 risk factors, median overall survival 27 months), and poor risk (3 or more risk factors, median overall survival 8.8 months) ( Fig. 1 ). Formal comparison of these angiogenesis-era models are under way. In the phase 3 trial evaluating temsirolimus, Hudes and colleagues defined poor-prognosis mRCC as the presence of at least 3 of the MSKCC criteria (see Table 2 ), KPS of 60 to 70, or metastases in multiple organs. Thus, some patients in the trial would have been considered intermediate risk by strict MSKCC criteria. At present, angiogenesis-era models are also used to classify patients treated with mTOR inhibitors.

These multivariable models are often used to analyze outcomes from clinical trials to determine the magnitude of benefit from novel therapies across the favorable, intermediate, and poor prognosis risk groups. It appears that all risk groups benefit from targeted therapies in prospective trials and retrospective population-based analyses. In the pivotal trial that demonstrated the superiority of sunitinib compared with interferon-α in mRCC, subgroup analyses revealed a significant benefit in progression-free survival (PFS) in the favorable and intermediate MSKCC risk groups. The poor risk group had too few patients to detect a difference in this phase 3 trial ; however, in a retrospective population-based analysis there appeared to be benefit in all risk groups. Thus, these prognostic-factor models are useful in estimating overall survival but may not be predictive of treatment benefit.
Biomarkers in renal cell carcinoma
A biomarker is a characteristic that can be objectively measured and evaluated as an indicator of a normal biological process, pathogenic process, or pharmacologic response to treatment. A variety of biomarkers has been evaluated in patients with RCC with the goal of individualizing cancer care and improving on the clinical prognostic-factor models. Markers associated with hypoxia and angiogenesis have been evaluated, for example, the Von Hippel-Lindau (VHL) pathway, hypoxia-inducible factor (HIF), VEGF family, carbonic anhydrase IX (CAIX), and the mammalian target of rapamycin (mTOR) pathway. Other promising markers include immune regulators, neutrophil gelatinase-associated lipocalin (NGAL), IMP3, thymidylate synthetase, and molecular expression profiling.
Von Hippel-Lindau Pathway
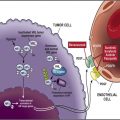
VHL is a tumor-suppressor gene located on chromosome 3p25 that encodes a gene product (pVHL). The VHL pathway plays a crucial role in adaptation to hypoxia ( Fig. 2 ), and functional loss of pVHL has been implicated in hereditary and sporadic ccRCC. In the absence of VHL, HIF is able to activate many downstream signaling pathways associated with angiogenesis, as detailed in Fig. 2 .
Von Hippel-Lindau Alterations
The frequency of VHL mutations in sporadic RCC has been estimated to be more than 42%. VHL mutations can occur via frameshift deletions/insertions, missense and nonsense mutations, loss of heterozygosity (LOH), and VHL promoter methylation. Exploration into the prognostic role of VHL gene mutation has produced conflicting results. Several groups have failed to demonstrate a relationship between VHL genetic alterations and survival. Others have suggested that VHL inactivation is associated with a more favorable outcome. Conversely, other studies found that loss of function mutations were associated with worse clinical outcomes. Thus the prognostic value of VHL gene mutation is not clear. Loss of function of pVHL is thought to be an early step in the carcinogenesis that is followed by further genetic alterations ; this may account for the lack of impact on prognosis. This area of study is limited by the available technology able to identify mutations or alterations in promoter methylation, as well as the possible influence of missense mutations that may not have functional significance.
Investigation into VHL mutation as a predictive factor has lagged behind the development of targeted therapy. In the realm of immunotherapy, a small study of patients with VHL alteration (n = 16) did not reveal any association with response to immunotherapy. Choueiri and colleagues evaluated VHL gene status in 123 patients with metastatic ccRCC who were treated with antiangiogenic therapies. Patients with loss-of-function mutations had higher response rates than those with wild-type VHL (relative risk [RR] 52% vs 31%, respectively; P = .04) and loss-of-function mutation was validated on multivariate analysis as an independent predictor of response. PFS and overall survival were not significantly influenced by VHL gene status or loss-of-function mutations, although at the time of publication only 40% of patients had died, making the survival data somewhat immature. VHL alterations were not associated with benefit from treatment with axitinib (n = 13), pazopanib (n = 63), or temsirolimus (n = 16). Prospective studies involving large, well-defined patient cohorts with standardized laboratory analysis are needed to further clarify this issue.
Hypoxia-Inducible Factor
HIF has been evaluated as a prognostic factor using immunohistochemistry (IHC) on tissue microarrays (TMAs). High cytoplasmic HIF-1α expression was associated with a trend toward improved survival by Lidgren and colleagues (n = 176 ccRCC); conversely, it was associated with significantly shorter survival in a smaller series analyzed by Dorevic and colleagues (n = 94). High nuclear HIF-1α expression (>35%) was associated with worse survival by Klatte and colleagues (n = 357), whereas Dorevic and colleagues found that it was associated with better survival. Patel and colleagues evaluated pretreatment HIF levels by Western analysis in 43 mRCC patients treated with sunitinib. The presence of high levels of HIF-1α ( P = .003) or HIF-2α ( P = .001) was associated with a higher likelihood of response. Figlin and colleagues analyzed IHC expression of HIF-1α and HIF-2α in a subset of patients with available archival paraffin-embedded tumor samples from Hudes’ randomized phase 3 trial of temsirolimus, interferon-α, or the combination, in advanced RCC with poor prognostic factors. Results for HIF-2α were not analyzed due to heterogeneous HIF-2α staining. There was no correlation between baseline HIF-1α levels with survival or response. At this time, the prognostic value of HIF-1α remains unclear.
Vascular Endothelial Growth Factor Family
The VEGF family includes multiple ligands (VEGF-A, B, C, D, and platelet-derived growth factor) as well as 3 tyrosine kinase receptors (VEGFR-1, 2, 3), which are part of the signaling pathways for angiogenesis and/or lymphangiogenesis. Given the intrinsic role of angiogenesis in the pathophysiology of RCC, a better understanding of these markers is expected to reveal important information about outcomes and therapeutic efficacy.
Patients with RCC have higher serum VEGF levels compared with healthy controls. On multivariate analysis, VEGF levels are not associated with overall survival in RCC patients treated with nephrectomy (n = 164).
For metastatic patients treated with immunotherapy, the results are conflicting. A study of 138 patients found no association (n = 138) ; however, serum VEGF was an independent prognostic factor in a larger study of 302 patients. The TARGET phase 3 trial, which randomized patients with mRCC to sorafenib or placebo, analyzed baseline VEGF levels in 712 patients. Higher VEGF levels significantly correlated with worse MSKCC score ( P <.0001) and worse ECOG performance status ( P <.0001). On multivariate analysis, higher baseline VEGF was an independent poor prognostic factor for PFS in the placebo arm, as well as overall survival for patients treated with placebo and sorafenib.
Baseline VEGF levels were not predictive of response from sorafenib in the TARGET trial, bevacizumab in the phase 3 AVOREN trial, the phase 2 trial of pazopanib, or patients treated with sunitinib.
Multiplatform Analysis
In a larger set of patients treated with pazopanib (n = 215), plasma levels of candidate markers (HGF, IL-6, IL-8, E-selectin, and VEGF) were assessed using 3 different platforms. Results from the 3 platforms were highly correlated and VEGF levels were not predictive. Lower baseline levels of HGF, IL-8, and IL-6 were significantly correlated with tumor shrinkage. Elevated levels of E-selectin, and lower levels of IL-6 and HGF were correlated with longer PFS. Large prospective trials are required to clarify the predictive role of these biomarkers.
Single-Nucleotide Polymorphism Analysis
Genomic DNA from 63 patients treated with sunitinib was analyzed by Kim and colleagues for single-nucleotide polymorphisms (SNPs) in VEGF (−2578, −1154, 936, −634) and VEGFR2 (889 and 1416). VEGF SNP 936 was associated with tumor shrinkage, and VEGFR2 SNPs were correlated with survival. Recently, Xu and colleagues analyzed 27 functional polymorphisms in angiogenesis and exposure-related genes from 241 patients treated with pazopanib. Polymorphisms in IL-8, FGFR2, VEGF (1154), FLT4, and NR112 were associated with overall survival ( P ≤.05). SNPs show promising potential as predictive and prognostic biomarkers, and further studies with larger numbers of patients are warranted.
Carbonic Anhydrase IX
CAIX is a transmembrane enzyme that might maintain a normal pH in hypoxic tumor cells, thereby fostering cancer growth and metastasis. CAIX expression, as assessed by IHC, is present in 94% to 97% of ccRCC. Bui and colleagues found that low CAIX expression (≤85%) was associated with worse overall survival for mRCC, but not for patients with localized disease using tissue microarrays (TMAs). Conversely, Sandlund and colleagues found that low CAIX expression was associated with poor prognosis in patients with Stage I to III disease, but not in patients with mRCC. Leibovich and colleagues conducted the largest retrospective study to date (n = 730) and found that low CAIX expression (≤85%) was not a significant prognostic factor on multivariate analysis. Leibovich’s group analyzed CAIX expression from whole tissue sections and noted significant intratumoral heterogeneity. Bui and colleagues observed that metastatic lesions had significantly lower CAIX staining levels compared with matched primary specimens; however, only 15 cases were analyzed. Thus, metastatic lesions may not be representative of the primary tumor. It is uncertain whether CAIX is a prognostic factor for RCC.
Tumor CAIX expression has also been evaluated as a predictive factor for patients with mRCC. Retrospective analyses suggested that high CAIX expression (>85%) was predictive of response to IL-2. A small study evaluated the impact of CAIX expression (IHC) and SNPs on outcomes of patients treated with IL-2 (n = 54). There was no association between SNPs and CAIX expression. The C allele variant of CA9 SNP rs12553173 and high CAIX expression (>85%) were both associated with increased response rates to IL-2, and were independent prognostic factors for overall survival. Conversely, in the prospective phase 2 SELECT trial, there was a trend toward higher response rates to IL-2 in patients with low CAIX (≤85%) compared with high CAIX (RR 38% vs 23%, P = .13).
Choueiri and colleagues evaluated tumor CAIX expression (IHC) in 94 patients treated with antiangiogenic therapies. CAIX expression was neither prognostic nor predictive of response to sunitinib. For sorafenib-treated patients, high CAIX expression (>85%) was associated with more tumor shrinkage compared with low CAIX expression (mean difference −22%). An analysis of 20 patients from a randomized phase 2 trial of temsirolimus in mRCC showed that CAIX expression (IHC) was not predictive of response. Further studies are required to evaluate CAIX as a predictive factor.
VHL Pathway Analysis
Analysis of VHL mutation status as well as plasma CAIX, VEGF, sVEGFR2, tissue inhibitor of metalloproteinase 1 (TIMP-1), and Ras p21 was performed in the TARGET trial of sorafenib versus placebo in advanced RCC. On multivariate analysis that included ECOG performance status, MSKCC score, and the biomarkers assayed, only baseline TIMP-1 levels were prognostic for survival ( P = .002). However, baseline TIMP-1 levels were available for only 123 patients. No predictive markers were identified.
Mammalian Target of Rapamycin Pathway
The mTOR pathway is downstream of the phosphoinositide-3-kinase (PI3K) and Akt pathway that is regulated by the phosphatase and tensin homolog (PTEN) tumor suppressor gene (see Fig. 2 ). Mutations in PTEN have not been found in RCC; however, diminished protein expression with increased levels of phosphoAkt (pAkt) have been observed. Activation of the mTOR pathway leads to activation of p70 S6 kinase (p70S6K), which phosphorylates the 40S ribosomal S6 protein (PS6) and upregulates HIF-1 gene expression. In patients with VHL mutations, mTOR activation can potentiate expression of HIF-inducible genes and promote cancer progression. PTEN, p70SK, and PS6 have been evaluated as pharmacodynamic markers of mTOR inhibition with temsirolimus (CCI-779) and everolimus (RAD001).
In vivo experiments have demonstrated that p70S6K inhibition by temsirolimus and everolimus are similar in peripheral blood mononuclear cells (PBMC) and tumor tissue. In 9 RCC patients treated with temsirolimus, there was a significant linear association between p70S6K activity inhibition in PBMCs (24 hours after treatment) and time to progression. The recommended phase 2 dose of everolimus was based on a pharmacokinetic/pharmacodynamic model using PBMC p70S6K inhibition. As mentioned previously, Cho and colleagues examined expression of CAIX, PS6, pAkt, and PTEN in paraffin-embedded tissue sections from 20 patients with advanced RCC treated with temsirolimus in a phase 2 clinical trial. These investigators found a positive association between PS6 expression ( P = .02) and a trend toward positive expression of pAKt ( P = .07) with objective response to temsirolimus. These results are early and require further evaluation.
Immune Regulators
Inhibition of T-cell mediated immunity has been shown to impair a host’s ability to generate a productive immune response against cancer. The B7 family consists of coregulatory molecules that inhibit T-cell mediated immunity. These molecules are normally present on monocyte-derived cells; however, aberrant expression has been linked to poor prognosis in RCC (see Table 3 ). Tumoral B7-H1 expression, as determined by IHC, has been evaluated in patients who underwent nephrectomy for ccRCC in a series of 196 fresh-frozen as well as 306 paraffin-embedded specimens. Positive expression of B7-H1 was associated with increased risk of death on multivariate analysis in both studies. Krambeck and colleagues analyzed tumoral B7-H4 expression using IHC on 259 fresh-frozen RCC nephrectomy specimens. Positive tumor B7-H4 expression was associated with an increased risk of cancer-related mortality on univariate analysis; however, this was not statistically significant after adjusting for SSIGN score. Cancer-specific survival rates were significantly lower for patients with coexpression of B7-H1 and B7-H4 on multivariate analysis after adjusting for SSIGN score ( P <.001). This result suggests that B7-H1 and B7-H4 may abrogate immune responses against RCC and that therapies that stimulate T-cell mediated immunity may be beneficial in RCC.
Signal transducer and activator 3 (STAT3) is a ligand-induced transcription factor that is activated in response to growth factors and cytokines, and is an important contributor to impaired antitumor immunity. Ito and colleagues analyzed 463 SNPs in 33 candidate genes from 75 Japanese patients treated with interferon-α for mRCC, and found that a STAT3 polymorphism was the most significant predictor of response (odds ratio 2.73). This work underscores the importance of the immune system in cytokine-based therapy for RCC, but has not been validated in other ethnic populations.
Neutrophil Gelatinase-Associated Lipocalin
Another potential predictive biomarker for patients with mRCC treated with sunitinib is NGAL. NGAL is an acute-phase protein linked to metalloproteinase-9 (MMP-9), which is involved in the degradation of the extracellular matrix, invasion, and metastasis. This protein induces a survival response and is upregulated in several human cancers (for a recent review see Ref. ). NGAL expression is present in clear-cell, papillary, and chromophobe RCCs. Porta and colleagues evaluated MSKCC score, baseline plasma VEGF, and NGAL titers in 85 patients with advanced RCC treated with sunitinib. On univariate analysis, baseline VEGF and NGAL were significant predictors of PFS, whereas MSKCC score was not. Patients with NGAL levels above 177 ng/mL had an RR of progressing of 1.86 (95% confidence interval [CI]: 1.142–3.019; P = .03) and a median PFS of 3.35 months (95% CI: 2.3–10.9) compared with 8.15 months (95% CI: 5.5–11.6) in patients with NGAL levels below this threshold. After adjusting the NGAL threshold to 110 ng/mL, both VEGF and NGAL maintained significance on bivariate and multivariate analysis. This study is another showing that baseline VEGF levels have prognostic potential and that molecular markers can outperform a clinical score (MSKCC score). The NGAL threshold established by this study requires external validation.
IMP3
IMP3 is a member of the insulin-like growth factor II (IGF-II) mRNA-binding protein family that is thought to regulate the production of IGF-II. It is expressed during embryogenesis, but its expression is virtually absent in normal adult tissues. Jiang and colleagues analyzed IMP3 IHC expression in 371 patients with localized primary RCC tumors. IMP3 status (positive vs negative) was a significant independent prognostic factor for overall survival, hazard ratio 4.01 (95% CI: 2.66–6.05; P <.0001), after multivariate adjustment for age, sex, tumor size, stage, grade, and histology. Using the same criteria for IMP3 assessment, the prognostic value of IMP3 was externally validated by Hoffmann and colleagues. IMP3 expression was present in 29.8% of ccRCC specimens (213/716). On multivariate analysis, positive IMP3 expression was associated with an increase in the risk of death from RCC (hazard ratio 1.42, P <.024). IMP3 has not been evaluated as a predictive marker.
Thymidylate Synthetase
S-1 is an oral combination of tegafur, a prodrug of fluorouracil (FU), and 2 other agents that act to maintain effective concentrations of FU in plasma and tumor while inhibiting the phosphorylation of FU in the gastrointestinal tract, minimizing gastrointestinal toxicity from FU. S-1 is widely used in Asia. A phase 2 study of 45 Japanese patients with cytokine refractory mRCC demonstrated a 24.4% objective response rate. Pharmacogenetic analysis of FU-related enzymes revealed that thymidylate synthetase (TS) expression was significantly lower in patients who responded to treatment ( P = .048), and PFS was significantly longer in patients with TS mRNA levels below the median level ( P = .006). There was no significant difference in response rate and overall survival between the low versus high TS group. No significant association was observed for the other FU-related enzymes that were analyzed. Intratumoral expression of TS mRNA is a promising predictive marker for benefit from S-1. FU-related gene polymorphisms should be evaluated prospectively in future randomized controlled trials of S-1 and in different ethnic populations.
Molecular Expression Profiling
Analysis of molecular expression profiles permits simultaneous measurement of thousands of genes to create a global picture of cellular function. Multiple groups have evaluated gene expression profiling in RCC, with variable results. Rini and colleagues. conducted the largest genomic study of 931 patients who underwent nephrectomy for localized ccRCC using reverse transcriptase quantitative polymerase chain reaction. From the 732 genes examined, they identified 16 genes significantly associated with relapse-free survival after adjustments for clinicopathological covariates and false discovery (hazard ratio 0.68–0.80). External validation of this promising prognostic multigene algorithm is warranted. Given the clinical utility of the 21-gene recurrence score for hormone receptor–positive, node-negative breast cancer (OncotypeDx), gene expression profiling has the potential to aid in risk stratification in localized RCC as well. It is essential that investigators adhere to rigorous statistical methodology to control for multiple testing and to minimize bias.
Biomarker Prognostic Models
The integration of multiple biomarkers and clinical variables into nomograms has the potential to provide more meaningful information on outcomes than either alone. Ki-67 is a marker of cell proliferation that has been associated with risk of cancer progression in multivariate ccRCC models. Kim and colleagues constructed a TMA from samples of 150 ccRCC patients and assessed IHC expression of 8 molecular markers: Ki67, p53, gelsolin, vimentin, epithelial cell adhesion molecule, CAIX, carbonic anhydrase XII, and PTEN. A prognostic model including CAIX, PTEN, vimentin, p53, tumor stage, and performance status was significantly more accurate than the UISS. Thus, analysis of several biomarkers permits evaluation of their relative prognostic utility. Parker and colleagues derived another biomarker-based scoring system from 634 patients with ccRCC. This weighted algorithm (BioScore) integrated dichotomized expression of B7-H1, Ki-67, and survivin. Patients with high BioScores (>4) were 5 times more likely to die from RCC than those with low scores. In addition, the sequential use (as opposed to integration into a new model) of BioScore with existing clinicopathologic scoring systems (TNM, UISS, SSIGN) further enhanced the predictive ability compared with each of these scoring systems alone. Prospective validation of these biomarker prognostic models is required before routine clinical use can come about.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree