Introduction
Diabetes mellitus is a major metabolic disorder. Nearly half a billion people are living with diabetes worldwide. International Diabetes Federation estimated that over four million people aged 20–79 years died from diabetes-related causes in 2019 . The global impact of diabetes is increasing considerably; the number of diabetic patients is expected to increase to 578 million (10.2%) in 2030 and 700 million (10.9%) in 2045 .
Type 2 diabetes is the most common form of diabetes, accounting for around 90% of all diabetes worldwide. The prevalence of type 2 diabetes is rising across all continents, associated with population aging, increasing urbanization, and economic development . Although there is considerable variation by region, the incidence of type 1 diabetes is increasing worldwide as well .
The long-term micro and macrovascular complications of diabetes can be present at diagnosis in type 2 diabetes and can be detected as early as five years after the onset of type 1 diabetes .
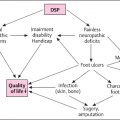
Morbidity and mortality associated with diabetes primarily result from chronic vascular complications. Complications of diabetes such as retinopathy, nephropathy, and cardiovascular diseases are leading to reduced quality of life, increased need for medical care, disability and decreased life expectancy in diabetic patients .
The overall prevalence of ay diabetic neuropathy was estimated to be 35% in diabetic patients . Chronic kidney disease resulting in diabetic nephropathy and other associated conditions was reported to be 25%–%36 in people with diabetes . Diabetic foot complications are associated with neurological disorders and peripheral vascular disease in the lower limbs .
The global prevalence of diabetic foot complications varies between 3% and 13%, with a global average of 6.4%. Lower limb amputation in people with diabetes is 10 to 20 times more common than those without diabetes .
There is a lack of understanding in the management and treatment of diabetic foot complications among healthcare professionals. Less than one-third of physicians recognize the signs of diabetic peripheral neuropathy. Increasing awareness of diabetic foot complications, risk assessments, and foot care by a multidisciplinary team can reduce foot complications and amputations by up to 85% .
Diabetes is the leading cause of neuropathy in the Western world. Diabetic neuropathies are the most common among the long-term complications of diabetes, affecting up to 50% of patients .
Epidemiologic studies indicate that neuropathy in diabetes patients is approximately 20% in community patients . An early study reported that incidence of neuropathy was 50% for patients with diabetes duration more than 25 years .
Diabetic neuropathy can cause significant morbidity and mortality. The major problem with Diabetic neuropathy is implicated in 50%–75% of nontraumatic amputations . Therefore it is essential to detect symptoms or signs of diabetic neuropathy and to determine risk factors as early as possible to implement interventions and prevent further neuronal damage.
In clinical practice, detection of diabetic neuropathy is challenging due to the lack of a clear consensus concerning the definition of the optimal clinical assessment criteria to diagnose neuropathy in diabetic patients.
Classification
Chronic hyperglycemia can affect any part of the nervous system. Sensory, autonomic, and motor neurons of the peripheral nervous system are vulnerable to the adverse effects of hyperglycemia in diabetic patients .
Various classifications for diabetic neuropathy have been proposed in recent years. Nevertheless, there is no universally accepted classification. Some of the suggested classifications are based on etiology, pathological or topographical features, and a classification based upon the clinical manifestation is most useful in clinical practice.
Thomas originally proposed the classification of neuropathy in diabetic patients in 1997, which most authors generally accept. Thomas divided diabetic neuropathies into four main groups: (1) Hyperglycemic neuropathy, (2) Symmetric polyneuropathy (Sensory/autonomic polyneuropathy, Acute painful diabetic neuropathy), (3) Focal and multifocal neuropathy (Cranial neuropathy, Thoraco-abdominal neuropathy, Focal limb neuropathies, Diabetic amyotrophy), (4) Mixed forms .
Recently the American Diabetes Association classified diabetic neuropathies into three main categories: (1) diffuse symmetric (distal symmetric polyneuropathy and autonomic), (2) mononeuropathy (mononeuropathy, mononeuritis multiplex, atypical forms), (3) radiculopathy, or polyradiculopathy .
Classification of diabetic neuropathy according to clinical presentation is shown in Table 1.1 .
| A. Generalized neuropathies |
|
| B. Focal neuropathies |
|
| C. Multifocal neuropathies |
|
Clinically, it is essential to distinguish between symmetric and asymmetric neuropathies because they require different diagnostic and therapeutic approaches. Although the most common neuropathy type is distal symmetric polyneuropathy in type 1 and type 2 diabetes, different forms of Diabetic neuropathy usually coexist in the same patient .
It is also essential to keep in mind that diabetes may not necessarily cause neuropathy in all diabetic patients. Other etiologic factors rather than diabetes need to be excluded before diagnosing diabetic neuropathy .
Risk factors
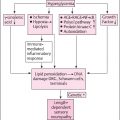
Hyperglycemia is the primary risk factor for diabetic neuropathy. Improved glycemic control can prevent the progression of diabetic neuropathy in type 1 diabetes but is not effective in preventing distal polyneuropathy in type 2 diabetes . Clinical trials have demonstrated the benefit of tight glucose control by slowing the progression of diabetic peripheral neuropathy in type 1 and type 2 diabetic patients . In an observational study long-term, persistent benefits of strict glycemic control were demonstrated in type 1 diabetic patients .
The duration of diabetes is a major risk factor for neuropathy, and it is well recognized that the risk of developing neuropathy increases with the duration of diabetes and age. As hyperglycemia takes time for nerve damage, diabetic neuropathy is more common in older adults (>50 years) .
Diabetic neuropathy should be suspected in all patients with type 2 diabetes and those who have had type 1 diabetes for more than five years .
Patients with type 2 diabetes develop distal symmetric polyneuropathy (DSPN) despite adequate glycemic control. The presence of multiple comorbidities such as hypertension, obesity, dyslipidemia might have attenuated the beneficial effects of glucose control.
Diabetic neuropathy can be increased by cardiovascular risk factors such as dyslipidemia, hypertriglyceridemia, low high-density lipoprotein, hypertension, abdominal obesity, independently from glycemic control. Obesity is associated with diabetic neuropathy in patients with Type 2 diabetes and in selected type 1 diabetic patients .
Population base studies showed that obesity is common in patients with neuropathy. Also, clinical studies revealed neuropathy was more prevalent in obese normoglycemic individuals than lean controls, suggesting obesity is an independent risk factor for neuropathy .
Observational studies highlighted that weight, Body mass index (BMI), and waist circumference are all associated with neuropathy. Obesity is the second most influential metabolic risk factor for neuropathy after diabetes .
Hypertension is a risk factor for diabetic neuropathy—it can be developed with diabetes progression or stimulate progression of chronic kidney disease. Essential hypertension can occur in diabetes patients as comorbidity, or diabetic nephropathy can cause secondary hypertension. Hypertension can aggravate polyneuropathies in diabetic patients as blood pressure control is emphasized clinically for distal symmetric polyneuropathy prevention .
Dyslipidemia is a common cardiovascular risk factor in diabetes. Hypertriglyceridemia was found to be an important independent risk factor corresponding to a 2.1-fold increase in distal symmetric polyneuropathy occurrence . According to the FIELD study, hypertriglyceridemia was a risk factor for new-onset DPN, and fenofibrate treatment reduced new-onset neuropathy by approximately 18% and reversed baseline neuropathy .
Height is considered as a marker for neuronal length, and it was reported as an independent predictor of neuropathy among patients with type 1 diabetes and type 2 diabetes .
Cigarette smoking and alcohol consumption are all considered independent risk factors for diabetic neuropathy . Most risk factors for diabetic neuropathy are modifiable and can be controlled or treated, while age, duration of diabetes, and height are not. Risk factors for diabetic neuropathy are summarized in Table 1.2 .
| Unmodifiable risk factors |
| Advanced Age |
| Duration of diabetes |
| Height |
| Modifiable risk factors |
| Poor glucose control (HbA1c, FPG) |
| Obesity (BMI, weight) |
| Abdominal Obesity |
| Dyslipidemia (high LDL, hypertriglyceridemia Low HDL) |
| Hypertension |
| Smoking |
| Heavy alcohol intake |
Symptoms and signs of diabetic neuropathy
The phenotype of diabetic neuropathy is heterogeneous with diverse clinical manifestations; some patients with diabetic neuropathy have few complaints, but their physical examination reveals mild to moderately severe sensory loss. At the other end, patients may have a severe neuropathic deficit with no symptoms .
Generalized diabetic neuropathy
Distal symmetric polyneuropathy
Distal symmetric polyneuropathy is the most common form among diabetic neuropathies accounting for about 75% of the diabetic neuropathies . The disease’s course is chronic and progressive. DSPN predisposes diabetic patients to variable degrees of pain, motor dysfunction, postural instability, gait abnormalities, nerve palsies, ulcers, burns, infections, gangrene, and Charcot’s disease. DSPN is the most common cause of foot ulceration and lower extremity amputation. It is also a major contributor to falls and fractures .
Neuropathic pain can cause physical and psychosocial impairment, disability, and reduced health-related quality of life. A minority number of patients are seen with anorexia, depression, and weight loss .
The development of DSPN is insidious, usually starting from the distal and progressing proximally to the extremities. Toes and under feet affected first, gradually ascending the leg. Fingertips and hand symptoms usually occur when distal extremity symptoms reach at knee level. Characteristic “stocking-glove” like distribution of neuropathic symptoms present in long-term diabetic patients .
Early symptoms occur in the DSPN disease course due to small nerve involvement followed by large nerve dysfunction. Small- and large-fiber dysfunction most commonly coexist.
DSPN manifests with either pain or gives rise to negative symptoms, but many patients experience both .
Common symptoms associated with small nerve dysfunction are pain and dysesthesias (unpleasant sensations of burning). Painful DSPN may also be described as a sensation of electricity, shooting pain, contact hyperalgesia, burning, lancinating, tingling, which tend to occur or worsen most at night . Pain evoked by contact, for example, with socks, shoes, and bedclothes (allodynia). Small fiber involvement might also give rise to negative symptoms, with selective loss of temperature and pain sensation . Large-fiber dysfunction is characterized by may cause numbness, tingling without pain, and loss of protective sensation is a risk factor for diabetic foot ulceration. Patients frequently complain that their feet feel like they are wrapped in wool or walk on thick socks. Large-fiber dysfunction is a risk factor of falls as the gait might be insecure, either wide-based or high stepping .
Autonomic neuropathy
Autonomic neuropathy may be observed at any stage of diabetes. Usually, it develops in patients who have had the disease for 20 years. The sympathetic, parasympathetic, and enteric nerves are affected in diabetic autonomic neuropathy .
Cardiovascular autonomic neuropathy is a crucial cause of morbidity and mortality in type 1 and type 2 diabetic patients. Its association with increased incidence of malignant arrhythmia and sudden death was demonstrated in diabetes .
In type 1 diabetes, autonomy imbalance may result in a prolonged Q.T. interval on the electrocardiogram, which may predispose the patient to life-threatening cardiac arrhythmias and sudden death . Diabetic neuropathy also can reduce appreciation of ischemic pain, which may delay the diagnosis of silent or asymptomatic myocardial infarction .
Clinical findings include exercise intolerance, fatigue, syncope or dizziness, persistent sinus tachycardia, decreased heart rate variability, bradycardia, supine hypertension. Vasomotor neuropathy frequently causes orthostatic hypotension . Orthostatic blood pressure measurements may be used to evaluate cardiovascular autonomic dysfunction .
Gastrointestinal autonomic neuropathy may cause paresis anywhere in the gastrointestinal system. The most common symptoms are gastroparesis, dysphagia, diabetic diarrhea, and constipation . Delayed esophageal transit and gastroparesis occur in approximately 50% and 40% of long-standing diabetics, respectively. Gastroparesis may promote dysphagia, regurgitation, esophageal erosion, and strictures . Delayed gastric emptying and gastric retention can cause early satiety, cramping, bloating, epigastric pain (heartburn), nausea and vomiting, and loss of appetite to the point of anorexia .
Delayed gastric emptying and gastric retention may impair medication absorption and prone patients to hypoglycemia . Colon abnormalities may result in severe constipation, diarrhea, and fecal incontinence . Symptoms of gastrointestinal autonomic neuropathy do not typically occur until later in the course of diabetes.
Genitourinary autonomic neuropathy affects sexual function and bladder problems which negatively affect quality-of-life issues in diabetes.
Erectile dysfunction can also be mediated by autonomic dysfunction and occurs in approximately 35% to 90% of men with diabetes . In women, diabetic neuropathy may cause vaginal dryness, dyspareunia, and reduced libido .
Bladder dysfunction is more frequently observed in type 1 diabetic patients than type 2 diabetes.
Impaired bladder sensation may result in retention and incomplete voiding of urine, overflow incontinence, and urinary tract infections . The most common symptoms of bladder dysfunction include dysuria, frequency, urgency, nocturia, incomplete voiding, and urinary incontinence.
Diabetic Sudomotor Dysfunction: Diabetic autonomic neuropathy initially results in a loss of thermoregulatory sweating, resulting in global anhidrosis. Diabetic autonomic neuropathy can also cause hyperhidrosis, Gustatory sweating, and abnormal sweat production that appears over the face, head, neck, shoulders, and chest after consuming food .
Symptoms and consequences of distal symmetric diabetic polyneuropathy and diabetic autonomic neuropathy are summarized in Table 1.3 .