Fig. 3.1
The intravasation and migration of CTC (Adapted from detection and clinical implications of circulating tumor cells (2015), Joosse SA et al. [1])
According to their biology characteristics, CTCs can be subdivided into two groups: epithelial-like and EMT-associated. Epithelial-like CTCs usually form from the primary tumor and enter circulation directly by forces from external side, such as friction, mechanical forces, or tumor growth [2]. This type of CTCs derived is more possible to remain its original phenotype. In this way, epithelial-specific marker detection such as the EpCAM (the epithelial cell adhesion molecule) will be feasible [6]. During the process of invasion, CTCs may undergo EMT (epithelial-to-mesenchymal transition), which increases their aggression and metastatic abilities. As such, this type of CTC has been classified as an EMT-associated CTC. Cells within this group have lost cell-to-cell adherence proteins such as cadherin and have subsequently gained the ability to migrate and invade [7].
3.3 Detection of Circulating Tumor Cells
3.3.1 Enrichment of Circulating Tumor Cells
CTCs are quite rare in circulating, making their exact quantification difficult. We may find only one CTC against the background of 106–107 normal peripheral mononuclear blood cells. In the patient without obvious metastatic mass, this occurrence rate may even be much lower. Such low numbers mean that systematic removal of PBMCs and selective enrichment of CTC are required when detecting CTCs in the blood. To date, more than 40 techniques have been developed for CTC detection, and novel strategies are still being published to date [8]. Generally speaking, there are five types of CTC isolation methods: (1) enrichment by density gradient centrifugation, (2) immune-magnetic separation (3) size-based isolation, (4) CTC-Chip, and (5) in vivo isolation (Fig. 3.2). We will now discuss each of these in more specific detail.
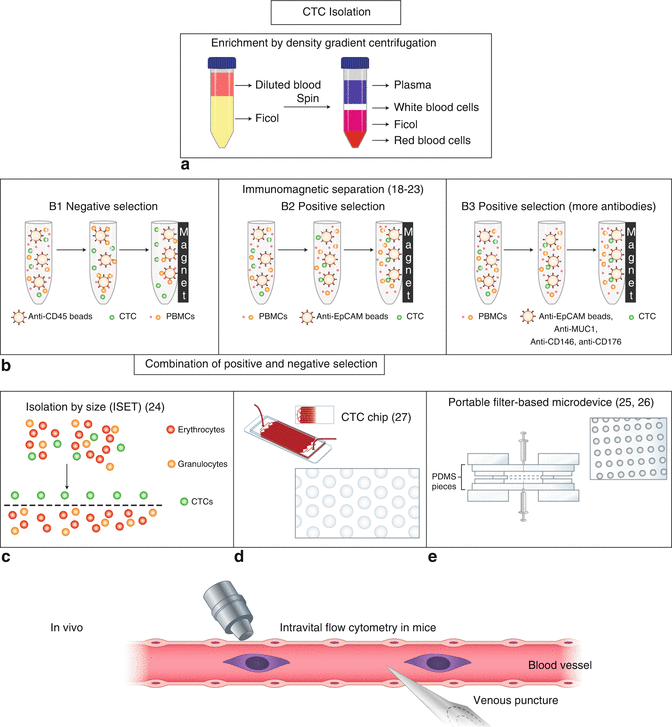

Fig. 3.2
In vitro and in vivo CTC isolation methods (Adapted from Circulating tumor cells in breast cancer: detection systems, molecular characterization, and future challenges (2011), Lianidou ES, Markou A [9])
3.3.1.1 Enrichment by Density Gradient Centrifugation
Density gradient centrifugation was the earliest technique to be developed. It remains the simplest way to isolate CTCs through the use of special centrifugation liquid. However, this method yields low rates of CTC enrichment. CD45 depletion can be combined with density gradient depending centrifugation to increase the yield of CTCs. It should be noted that density gradient depending centrifugation and lysis of red blood cell may cause the loss of tumor cell.
3.3.1.2 Immune-Magnetic Separation
A great many CTC detection technologies are immune-magnetic based, which can be divided into those that rely on positive selection and those that rely on negative selection. EpCAM is the most commonly used label in positive selection methodologies. CellSearch system is one of the EpCAM-based CTC detection technologies and is the only approved CTC detection apparatus by FDA (US Food and Drug Administration). By using this approach, CTC has been proven as an independent prognostic biomarker on PFS (progression-free survival) and OS (overall survival) in a variety of cancers, including lung, liver, colon, breast, and prostate [8, 10–14]. The AdnaTest is also epithelial marker-based and can enrich CTCs positively. A study on metastatic breast cancer used this method and proved that CTC presence or absence was a valid prognostic and predictive marker [15]. A major drawback of this method is that it is not perfect in detecting MET-associated CTCs, since these have either downregulated EpCAM or a complete loss of it [16].
CD45 is the most commonly used label for negative selection approaches. Depletion of leukocytes with CD45-positive markers is the favorite approach for capturing CTCs that have downregulated or an absence of EpCAM expression [17]. CD45 depletion could also be used together with other label-independent method, such as density gradient depending centrifugation to increase the yield.
3.3.1.3 Size-Based Isolation
The ISET system is a typical size-based CTC isolation device. In general, this approach is designed to gain CTCs at a high enrichment rate. However, a major drawback of this method is that EMT-associated CTC might be larger than leukocytes or not solid enough. As such, they may get loss by the size-based filter. What’s more, even though CTC is captured on the membrane, it might be difficult to detect and examine for further genetic analysis.
3.3.1.4 CTC-Chip
Microfluidic device platforms like the CTC-Chip are promising alternatives to selectively capture CTCs [18]. Quite a large number of CTC-Chips are currently available, including the IsoFlux for EpCAM-positive tumor selection [19], CTC-iChip (a size-based filtration and affinity-based enrichment strategy) for systematic removal of PBMCs and RBCs [20], JETTATM microfluidic chip (a size- and deformability-based capture scheme) enabling single-cell analysis [21], and the spiral biochip (a size-based and EpCAM-independent separation method) with low leukocyte contamination [22]. Generally speaking, CTC-Chips are advantageous in that they have high recovery rates; however they are inconvenient to use and come at a high cost.
3.3.1.5 In Vivo Isolation
To circumvent sample volume limitations, an EpCAM-coated wire named CellCollectorTM was designed by GILUPI GmbH for in vivo CTC capture [23]. This device is positioned through a cannula into the vein of a cancer patient. During the 30-min application time, it is estimated that up to 1.5 L of blood flows over the detector, thus increasing the yield of detectable CTCs. A similar device employing 3-aminopropyltriethoxysilane (γ-APS) as the coupling reagent and CBMA-grafted anti-EpCAM antibody-immobilized Nylon as cannula has also been developed. This method has also been shown to be a promising new material for in vivo CTC capture [24].
In general, CTC isolation can be achieved with different strategies. A great number of approaches for CTC enrichment are currently under development and may be on the market in the near future. However, both independent and large clinical studies will be needed to determine the robustness and clinical validity of these new methods.
3.3.2 Identification and Characterization of CTCs
CTCs could provide important information regarding disease progression as well as useful guidance for therapeutic strategies. Differential methods can now be applied to identify different types of CTC. To our knowledge, those methods include immunocytochemistry-based identification [25, 26], PCR-based identification [27–29], and EPISPOT-based identification [30, 31]. To this end, each strategy has its own advantages and disadvantages. As such, a combination of different analytical methodologies is likely to be beneficial and could allow for a better understanding of the role CTCs play in metastasis formation.
3.4 Ex Vivo and In Vivo Culture of CTCs
Past studies have shown that CTC is valuable predictive marker for disease recurrence prediction and useful prognostic marker for patient survival in quite a number of solid tumors [32–34]. However, it is also emphasized that not every CTC could lead to metastatic mass. Given this difference in metastatic capacity, it is quite important to figure out those CTCs which are able to migrate and form metastasis. This would then allow clinicians to target the formers specifically. Moreover, identifying CTCs with metastasis-initiating capabilities would bring a promising novel therapeutic target. To achieve this, one would need a thorough molecular and genetic characterization of CTCs from early cancers [35]. Moreover, if CTCs could be isolated, cultured, genotyped, and characterized through the whole course of therapeutic progress, they would allow clinicians to identify the most effective treatment strategy [36, 37].
3.4.1 Ex Vivo Culture of CTCs
Ten years ago, development of the EPISPOT assay allowed for the possibility of short-term cultures of CTCs. This assay is designed to detect specific proteins that are produced by CTCs during the culture in vitro. Studies in colon and breast tumor have indicated that the number of CTC is correlated with worse outcome for patients [31]. The culture of CTCs in vitro was first described by Zhang et al. in breast cancer [38].
In the study conducted by Zhang et al. [38], researchers characterized and developed an invasive and metastatic type of CTC in vitro, named BMSM, from breast cancer patients. In their study, they successfully isolated and cultured CTCs for long term and demonstrated CTC’s competency in the brain metastases [39]. In the Yu et al. study [40], a microfluidic technology termed CTC-iChip was reported. Using this chip and multiple rounds of testing with different culture conditions, they found that CTCs can proliferate better if cultured in serum-free media at tumor spheres, when supplemented with EGF (epidermal growth factor) and FGF (basic fibroblast growth factor) under hypoxic conditions.
In another study [41], a device based on immunoaffinity and microfluid was utilized and applied for lung cancer in early stage. In this study, researchers isolated and cultured CTC in the chip directly. And different culture environment was adopted to get the best conditions for the CTC’s growing. As a result, it is observed that CTCs grown at the co-culture environment in 3D could exhibit the best levels of expansion (Fig. 3.3). Given this finding, fibroblasts together with ECM (extracellular matrix) protein were applied to establish a tumor-related microenvironment to facilitate CTCs’ expansion. This study demonstrated that CTCs even in rare number got from cancer in early stage can also proliferate for further studies. Moreover, they could be used for the cancer-related gene sequencing without amplification previously, thus making gene study of CTCs more convenient.
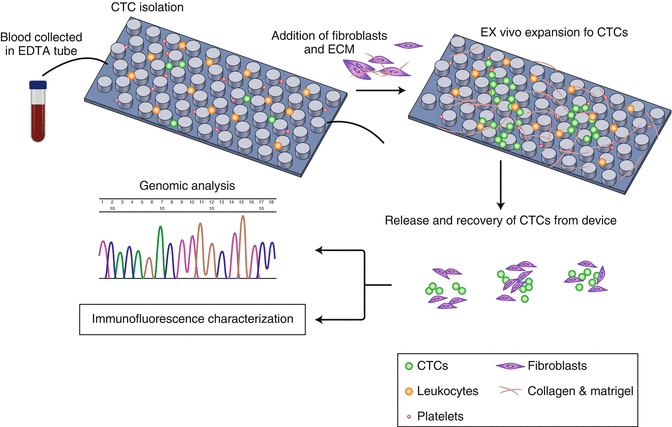

Fig. 3.3
Strategy for co-culture environment in vitro expansion of CTC (Adapted from Expansion of CTCs from early stage lung cancer patients using a microfluidic co-culture model (2014), Zhang Z et al. [41])
3.4.2 In Vivo Culture of CTC
Another method to enlarge the number of CTC is the xenotransplantation of CTCs derived from patients into immunodeficient mice. This method was first reported by Baccelli’s team and demonstrated that after xenotransplantation of CTCs from breast cancer patients into the bone of immunocompromised mice, they could grow and metastasize, with a phenotype of CD47+, CD44+, EpCAM−, and C-met+ [38].
The second report of in vivo cultured of CTCs was carried out on small-cell lung cancer patients. It was proved that the CTC from patients could be successfully xenotransplanted and tumorigenic in immunodeficient mice. Moreover, the CTC-derived explants could mirror the patients’ responses to etoposide and platinum chemosensitivity, which may help to provide useful information for chemo agents’ selection [42].
Recently, Cayrefourcq et al. reported the construction of CTC culture environment in vivo and their tumor-related characteristics in immunocompromised mice. CTCs from 71 colon cancer patients were tested by CellSearch device. Long-term CTC cultures were successfully enriched from two colon patients with a CTC number of more than 300. This was the first published report demonstrating that CTC detected in the peripheral blood of patients with metastatic colon cancer can be expanded and established as a stable colon cancer cell line. Further characteristic at the genomic and post-genomic level of this colon cell line revealed a special feature with attractive potent [43].
3.5 Clinical Implications of Circulating Tumor Cells
3.5.1 Prognostic Potentials of CTC in Gastric Cancer
Past studies have indicated that CTC is an important predictive and prognostic biomarker for the survival and recurrence of solid tumor patients. CTCs were proved to be a reliable biomarker for monitoring the recurrence earlier than radiographic image. By the time metastasis is significant radiographically, the size of tumor will be much larger to treat. Studies of advanced gastric cancer and other malignant tumor have shown that increased number of CTCs was correlated with worse survival [44]. To this end, a certain number of researchers are currently discovering and demonstrating the value of CTCs in clinical treatment and tumor burden monitoring.
One such study prospectively evaluated CTCs using CellSearch in advanced gastric cancer samples from 136 patients to decide the CTCs’ positivity frequency. The positive detection rate of CTC was 18.4%. Positive CTC count was much more frequent in the tumor of diffuse histologic genotype, as well as distant metastasis. A multivariate analysis revealed that CTCs could be regarded as independent factors for PFS in gastric tumor [44].
In another study, CTCs were isolated from 100 patients with advanced gastric cancer using the CTC-Profiler (Veridex). This isolation method is magnetic particle-based and uses a coating of anti-EpCAM antibody. The positive CTC detection rate was 28%. The treatment response, PFS, and OS in the CTC-positive group were significantly less than that of the CTC-negative group. Further multivariate analysis proved that CTCs were independent factors for PFS or OS in advanced gastric cancer [45].
A study conducted by Kolostova et al. established a new size-based separation enrichment (MetaCell®) and CTC cultivation approach. The positive CTC detection rate using this method was found to be 59% (n = 13/22). In addition to further cytomorphological analysis of the enriched CTCs, a gene expression analysis of tumor-associated genes was also performed [46].
Finally, another study has focused on the capacity of CTCs’ tumorigenicity in 42 patients with advanced gastric cancer. CTCs were separated and xenotransplanted into immunocompromised mice. After five months, nine tumor-like structures derived from six patients were established. These structures were also durable for passages [47].
3.5.2 Genetic Analysis of CTC
The analysis of CTCs’ molecular characterization could be useful in identifying therapeutic targets, thereby contributing to a more tailored, anti-metastatic therapy for each patient. These CTC-specific analyses included tumor-associated amplification, genomic tumor mutation profiling, detection of tumor-associated mRNA, and single-cell RNA sequencing. Single-cell-RNA sequencing of CTC has been reported in both breast [48] and pancreatic cancers [49]. Until now, there has been no single-cell RNA sequencing of either CTC clusters or single CTCs in gastric cancer.
3.5.2.1 Detection of Tumor-Associated Amplification in CTC
Recently, a new cellular- and molecular-based method of subtraction enrichment and immunostaining-based FISH (iFISH) was successfully established. The method promised effective enrichment, phenotypic identification in situ, and CTCs’ subtype characterization. This fine-grained information is critical, since different CTCs’ subtypes might contribute different clinical significances to tumor growth, relapse, metastasis, and treatment resistance [50].
A similar study was also carried out by Shen et al. Briefly, iFISH was also used to determine CTCs’ number and characteristics in advanced gastric cancer patients. The status of HER2 expression and the aneuploidy of the chromosome 8 in CTCs got from the patients were tested. Their results showed that examination of the CTC chromosome 8 copy number provided an important method for predicting chemosensitivity and monitoring treatment efficiency [51]. A similar study has also been conducted in breast cancer [52].
3.5.2.2 Genomic Tumor Mutation Profile Assessed in CTC
CTC analysis gnomically can also provide genetic mutation related to therapeutic resistance. For example, the mutations of KRAS were known to affect the efficiency of EGFR inhibitors in colorectal and lung cancer patients. An analysis of intra-patient KRAS [53] and BRAF mutation [54] heterogeneities in individual CTCs has been reported in colon cancer. The testing methods used included HRM, ASPCR, and ddPCR. The heterogeneity of PIK3CA mutation status was also investigated through single CTC detection in metastasis breast cancer patients [55]. Evaluation of PIK3CA mutational status in CTC could be a potential clinical method for tumor and treatment monitoring [56].Yet till now, no similar mutation reports are currently available in gastric cancer.
3.5.2.3 Detection of Tumor-Associated mRNA in CTC
Studies that have examined tumor-associated mRNA in CTC are rare. In a recent colon cancer study, CTC-specific mRNAs were investigated and shown to have higher expression in patients with ≥3 CTCs. mRNA expression levels of KRT19, KRT20, and AGR2 have also been reported in mCRC, but no similar results have been available in gastric cancer until recently [57].
3.6 Perspectives
In conclusion, CTCs obtained from circulation could be regarded as liquid biopsy and have important potential for the understanding of tumor metastasis biology. Moreover, using CTCs also lends itself to future improvements in the management of metastatic diseases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree