Toxic-metabolic
• Alcohol consumption
• Tobacco smoking
• Hypercalcemia
• Hyperlipidemia
• Chronic renal failure
• Medication and toxins (organotin compounds)
Idiopathic
• Tropical calcific pancreatitis
• Fibrocalculous pancreatitis
• Other
Genetic
• Cationic trypsinogen (R122H)
• CFTR mutation
• SPINK1 mutation
• α1-antitrypsinogen mutation
Autoimmune
• Autoimmune pancreatitis
• Sjogren’s syndrome
• Inflammatory bowel disease
• Primary biliary cirrhosis
Recurrent severe acute pancreatitis
• Postcomplicated severe acute pancreatitis
• Postradiation pancreatitis
• Ischemic pancreatitis
Obstructive
• Pancreatic divisum
• Sphincter of Oddi dysfunction
• Duct obstruction
• Periamullary and periductal tumors
• Pancreatic duct structures
Pathophysiology
Initiation of pancreatic injury differs between patients, however most follow common pathways, which include occlusion of ducts by external compression or protein plugs, leading to ductal and parenchymal hypertension, with resultant inflammation and fibrosis [5]. Often the onset of chronic pancreatitis is attributed to residual morphological pancreatic changes in the pancreas following an episode of acute pancreatitis, leading to alterations in the normal flow of pancreatic secretions [6]. Pancreatic duct pressures can rise from 7–15 mm Hg in healthy individuals to 80 mm Hg in severe cases. Ductal obstruction can occur in side branches of the pancreatic duct or at any point along the main duct. Ductal hypertension is attributed to ongoing exocrine secretion from pancreatic acini in conjunction with surrounding inelastic pancreatic parenchyma and capsule [7].
It has been long believed that relief of ductal hypertension by decompression of a dilated duct will relieve chronic pancreatitis-related pain [8]. However, the pathogenesis of pain, the major feature of chronic pancreatitis, is incompletely understood. Lack of any single successful therapeutic modality for CP pain suggests multifactorial and collusive mechanisms. Pancreatic sensation is derived from retroperitoneal autonomic nerves synapsing at the celiac ganglia, forming a neural network embedded in the periaortic fatty areolar tissue near the celiac and superior mesenteric arteries. Chronic pain levels correlate with perineural fibrosis and inflammatory cell infiltrates [9]. Increases in neural tissue, as well as hypertrophy from inflammatory infiltration, leave focal disruptions in the perineural sheath (which protects the nerves from inflammatory injury). Chronic stimulation of nociceptors with a concomitant decreased nerve stimulation threshold enhances responsiveness of nerves to stimuli and thus, increased pain.
Other mechanisms of pancreas injury include pancreatic ischemia, duct disruption, autodigestion by pancreatic enzymes, reactive oxygen species and oxidative injury, and activation of pancreatic stellate cells leading to fibrosis. Progressive loss of exocrine and endocrine function is often accompanied by unrelenting or recurrent pain requiring medical and/or surgical intervention. It is often observed that progressive cases of chronic pancreatitis may “burn out” after many decades, however this phenomenon is unpredictable and pain often persists despite loss of function.
Marseille, Cambridge, and Rosemont Classification Systems
An international consensus classification for inflammatory disease of the pancreas in use since 1963, termed the Marseille classification [10], groups pancreatitis patients into four main groups: (1) acute; (2) relapsing acute with no residual glandular injury; (3) chronic relapsing, characterized by pain and acute exacerbations; and (4) chronic, with progressive functional damage to the gland. Since that time, new technology has made anatomical and radiological determinations available to provide more sophisticated classification schemes [11].
While pancreatogram findings do not always correlate to disease pathology or patient symptoms, the popular Cambridge classification, first introduced in 1984, employs ERCP, ultrasound, or CT imaging to classify the severity of disease. Changes in MPD caliber and parenchymal changes (summarized in Table 30.2) stratify patients from normal to severe (Cambridge class III) pancreatitis [12].
Table 30.2
Cambridge classification for chronic pancreatitis
Cambridge class 0 | |
Severity | Equivocal |
ERCP | Less than three abnormal side branches |
Imaging | MPD 2–4 mm, gland 1–2 × normal size |
Cambridge class I | |
Severity | Mild |
ERCP | 3 or more abnormal side branches |
Imaging | Cavities < 10 mm, MPD irregularity, focal acute necrosis, parenchymal heterogeneity, hyperechoic duct wall, contour irregularity of the gland |
Cambridge class II | |
Severity | Moderate |
ERCP | More than 3 abnormal side branches and abnomal MPD |
Imaging | Cavities < 10 mm, MPD irregularity, focal acute necrosis, parenchymal heterogeneity, hyperechoic duct wall, contour irregularity of the gland |
Cambridge class III | |
Severity | Severe |
ERCP | >3 abnormal side branches + abnomal MPD + at least one additional finding (large cavity > 10 mm, intraductal filling defect, duct obstruction, or ductal dilation) |
Imaging | Cavities < 10 mm, MPD irregularity, focal acute necrosis, parenchymal heterogeneity, hyperechoic duct wall, contour irregularity of the gland + at least one additional finding (large cavity > 10 mm, intraductal filling defect, duct obstruction, or ductal dilation, calcifications, organ invasion) |
Since the 1980s, endoscopic ultrasound has become an increasingly valuable tool to visualize changes in pancreatic parenchyma and duct, however the interobserver agreement for the diagnosis of chronic pancreatitis was highly variable. Previous diagnostic criteria typically required the presence of three to five features out of nine, and equally weighted endoscopic findings consistent with chronic pancreatitis [13]. Three or more endoscopic features have 83% sensitivity and 80% specificity for the diagnosis of chronic pancreatitis, with predictably higher specificity with more stringent requirements [14, 15]. A consensus statement introduced in 2009 termed the “Rosemont criteria” proposed standardized definitions and criteria for the diagnosis of chronic pancreatitis by endoscopic ultrasound (summarized in Table 30.3) [16].
Table 30.3
Rosemont criteria for diagnosis of chronic pancreatitis
Rosemont endoscopic features of CP | ||
Major A features | Hyperechoic foci (≥2 mm) | |
MPD calculi | ||
Major B features | Lobularity or honeycombing of parenchyma | |
Minor features | Anechoic Cysts | |
Hyperechoic stranding (≥3 mm) | ||
Hyperechoic foci (≥2 mm) | ||
Dilated duct (≥3.5 mm in body, ≥1.5 in tail) | ||
Irregular MPD contour | ||
≥3 dilated side branches (≥1 mm) | ||
Hyperechoic MPD wall | ||
Rosemont criteria for diagnosis of CP | ||
Consistent with CP | 1 major A feature | AND ≥ 3 minor features |
1 major A feature | AND 1 major B feature | |
2 major A features alone | ||
Suggestive of CP | 1 major A feature | AND < 3 minor features |
1 major B feature | AND ≥ 3 minor features | |
≥5 minor features | ||
Indeterminate for CP | 3–4 minor features alone | |
major B feature alone | AND < 3 minor features | |
Normal | 1 or 2 minor features | |
Case Presentation: Surgical Treatment of Chronic Pancreatitis
A 42-year-old gentleman with a history of long-standing alcohol abuse and gallstone pancreatitis 2 years prior presents to the emergency department with complaints of abdominal pain. He reports his symptoms were provoked by alcohol consumption 1 day prior, and radiates bilaterally from the epigastric region to the spine. He also endorses nausea and anorexia. Review of his medical history reveals two prior episodes of self-limited abdominal pain in the current year, with no other contributing medical, surgical, or social comorbidities.
Differential Diagnosis
A broad differential diagnosis for the case of acute or recurrent epigastric abdominal pain includes, but is not limited to, the following:
Pancreatitis
Peptic ulcer disease
Gastritis
Intestinal obstruction
Mesenteric ischemia
Cardiac and pleural sources
In the United States, alcohol abuse continues to be the prominent risk factor for chronic pancreatitis, responsible for 45% of cases [17]. Chronic pancreatitis attributed to alcohol consumption usually presents in the fourth to sixth decades of life, and correlates with cumulative alcohol intake. While the patient in this case presented with a history of heavy alcohol intake, there is no safe level of alcohol consumption with regard to risk of chronic pancreatitis [18, 19]. A minority of alcoholics will develop alcoholic chronic pancreatitis, suggesting a genetic predisposition or other susceptibility in some patients [20].
A majority of cases of chronic pancreatitis not related to alcohol use are idiopathic. The presentation of idiopathic chronic pancreatitis has a bimodal age distribution, with an early onset group presenting during adolescence, and another late-onset group occurring at a mean age of 56 years. Pain is almost uniformly the first symptom in early onset chronic pancreatitis. Some cases can be attributed to an initial episode of severe acute pancreatitis, sometimes called the sentinel acute pancreatitis event, or SAPE. Late-onset disease is commonly associated with pain, however episodes of severe pain are less frequently reported [21].
The natural history of pain in all types of pancreatitis improves in severity and frequency in approximately two-thirds of cases. In a minority of cases, pain becomes worse over time. The course of progression in alcoholic chronic pancreatitis is reciprocal to the degree of alcohol abstinence. The pattern of recurrent pain also appears to follow two distinct patterns. In its early course, symptoms are akin to recurrent episodes of acute pancreatitis, with prolonged periods of absence between episodes. In later stages, many develop a “smoldering” pancreatitis, with episodes of exacerbation and associated endocrine dysfunction [22]. A sizable number of cases reach a terminal “burn out” of chronic pancreatitis, in which pain is improved or absent, and exocrine/endocrine deterioration comes to a halt [23, 24].
Long-standing chronic pancreatitis often leads to local complications responsible for significant additional morbidity. Intraductal calculi (pancreatolithiasis) and ductal stenosis are common and lead to duct dilation. Splenic vein thrombosis can occur due to its close proximity to the inflammatory process, and leads to hypersplenism and gastric varices. Pancreatic pseudocysts are common and frequently cause pain, portal vein compression, and sometimes even intestinal obstruction. Rarely, erosion into major vascular structures can lead to life-threatening hemorrhage. The risk of malignancy arising from a background of chronic pancreatitis is elevated four to six times that of the general population; however, the absolute risk is still low and may not warrant additional screening. In some cases, ductal dilation is accompanied by fibrosis and phlegmon of the pancreatic head. This can be concerning not only due to mass effect on surrounding structures, but also in shrouding the diagnosis of pancreatic head malignancies.
Technical Pearls
Identifying a dilated pancreatic duct with a small-gauge needle and syringe, with or without ultrasound guidance, can help minimize blind attempts in finding the duct, and minimizing chances of unnecessary trauma to the pancreas.
Ensure the Roux limb is at least 40 to 60 cm in length, to minimize the risk of gastrointestinal content reflux into the pancreaticojejunostomy.
When performing a Frey procedure, ensure that the SMV and portal vein are clearly identified so as to minimize the risk of inadvertent injury.
When performing a Frey procedure, perform a full Kocher maneuver to appreciate the thickness of the pancreatic head, and minimize the chance of full-thickness debridement.
Workup
Eliciting a history of recurrent abdominal pain, anorexia, and nausea which is exacerbated by food intake should provoke an investigation toward pancreatitis as a possible diagnosis. Patients may also exhibit hyperglycemia, jaundice, steatorrhea, weight loss, and endorse a history of heavy alcohol use. Symptoms are variable, and up to 20% of patients with pancreatitis will present with “painless pancreatitis.” Initial laboratory testing should include serum amylase and lipase levels, however these markers may lose sensitivity after extensive glandular injury has impaired exocrine enzyme production. Extensive fibrosis and edema may lead to elevation of serum bilirubin and alkaline phosphatase in a minority of patients. Additionally, fecal fat excretion can be detected (alternatively Sudan staining of the feces can be utilized), although it is neither sensitive nor specific. Fecal chymotrypsin, trypsin, and elastase testing are now widely available, but suffer from low sensitivity in all but very late-stage disease. Enhanced pancreatic function tests have been studied, including MRCP and endoscopic-assisted secretin stimulation testing, but these techniques have yet to be widely adopted [25].
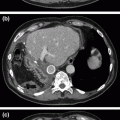
Endoscopic retrograde pancreatography (ERCP) was classically the reference imaging modality for the diagnosis of chronic pancreatitis, however, increasing consensus is developing around the use of endoscopic ultrasound (EUS) criteria. Studies comparing EUS to ERCP in the diagnosis of chronic pancreatitis have found good correlation between morphologic abnormalities seen on EUS with a sensitivity of 85–97% [26, 27]. However, in practice, computed tomography is almost always included as a powerful adjunct; modern CT scanning has 60–90% sensitivity, as well as 85–95% specificity in the diagnosis of chronic pancreatitis, with increasing sensitivity in more advanced disease [27, 28]. CT findings in chronic pancreatitis include parenchymal atrophy, inflammatory changes, calcifications, pseudocysts, and pancreatic and bile duct dilation. Irregular ductal contour, strictures, and side-branch abnormalities may be more prominent on ERCP and assist in classification and treatment planning. Transabdominal ultrasonography and plain films may detect large cysts, ductal dilation, and calcification, but are rarely used in guidance of treatment algorithms. Magnetic resonance imaging, while promising, is not clearly superior to ERCP and CT [5, 29].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree