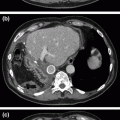
Fig. 31.1
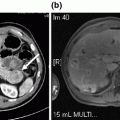
CT abdomen with IV/PO contrast showing a extensive dystrophic calcifications throughout the pancreatic head with diffuse dilation of b the proximal main-pancreatic duct (9 mm), with an intra-pancreatic duct calculus associated with mild edema and peri-pancreatic stranding suggestive of acute on chronic pancreatitis. c coronal image of the pancreatic head with calculi and dilated duct. d pancreatic duct dilation extends to the body and tail
Diagnosis and Assessment
Chronic pancreatitis is a fibro-inflammatory syndrome of the pancreas provoked by inflammatory or stress conditions incited through genetic and/or environmental risk factors, leading to morphologic (parenchymal injury, irregular fibrosis) and physiologic (exocrine and endocrine) changes with acinar and islet cell loss [1–5]. The pathophysiology of chronic pancreatitis is multifactorial and complex. The TIGAR-O (toxic/metabolic, idiopathic, genetic, autoimmune, recurrent pancreatitis, obstructive) classification system identifies risk-factor categories associated with chronic pancreatitis [1].
Our patient demonstrates a common presentation of chronic pancreatitis with chronic alcohol and tobacco abuse, recurrent acute pancreatitis, post-prandial abdominal pain, malabsorption (exocrine insufficiency), and weight loss. His exposure to long-term use of alcohol and tobacco places him at an elevated risk for pancreatic disease development and progression [6–9]. The correlation between alcohol use and pancreatic disease is common worldwide, including the United States [10]. Recurrent acute pancreatitis attacks destroy parenchymal tissue over time through chronic inflammatory changes, including fibrosis and calcification, leading to impaired exocrine and endocrine functions [11]. Chronic pancreatitis in the advanced phases is associated with an increased risk for pancreatic cancer [12–14]. Exocrine insufficiency largely takes place when more than 90% of acinar cell function is lost leading to malabsorption [15]. Steatorrhea (fat malabsorption) precedes azotorrhea (protein malabsorption), leading to weight loss over months to years, as seen in our patient. Patients commonly present with boring epigastric pain radiating to the back in a band-like fashion. Pancreatic pseudocyst formation (not found in our patient) may also cause pain, early satiety, weight loss, and inability to eat, resulting in weight loss in the more acute setting.
Initial workup should include well-validated radiologic imaging, typically with CT, ERCP, endoscopic ultrasound (EUS) with biopsy, or magnetic resonance cholangiopancreatography (MRCP). CT imaging with a pancreas protocol (with and without IV contrast, IV contrast in arterial and portal venous phases, and water as oral contrast) is the first-line noninvasive imaging modality of choice, and our initial gold standard. Studies using this protocol are able to diagnose chronic pancreatitis and its complications with 90% confidence [1]. Endoscopic methods of imaging with ERCP and EUS can provide imaging and tissue for diagnosis with therapeutic intervention using ERCP technique. The risks of bleeding, bowel or duct perforation, and acute pancreatitis must be taken into account with perceived benefits on a case-by-case basis. It is essential to assess the pancreatic and biliary duct anatomy for evidence of obstruction preoperatively to optimize intraoperative intervention.
In terms of pancreatic exocrine dysfunction, functional testing is invasive and not necessarily diagnostic, and should have a limited role in chronic pancreatitis workup. Instead, history and clinical evidence of steatorrhea/azotorrhea with or without weight loss may be a signal of malabsorption and a malnourished, catabolic state. It is critical to evaluate the patient’s nutritional status preoperatively through a metabolic and nutritional panel not limited to fat-soluble vitamins, liver function tests, pre-albumin, and albumin. Malnourished patients need nasojejunal enteral feedings for 2–4 weeks to reestablish an anabolic state to prepare for surgery.
Patients with chronic pancreatitis often present with glucose intolerance from endocrine insufficiency, as up to 60% of patients will require insulin replacement [16]. Further, chronic pancreatitis patients are at an increased risk for spontaneous or treatment-related hypoglycemia, likely attributed to glucagon insufficiency, malnutrition, and alcohol consumption [16]. Our patient did not have endocrine insufficiency.
Management
Whenever feasible, lifestyle modifications should be implemented for chronic pancreatitis patients prior to surgical intervention. In addition to multimodal pain therapies, alcohol and smoking cessation can further decrease pain and complications in chronic pancreatitis, with further risk mitigation of pancreatic calcification through smoking cessation [17–19]. Reductions in dietary fats for patients with severe refractory steatorrhea offer some benefit, yet medium-chain triglyceride supplementation are not indicated and require additional enzyme supplementation for proper digestion and absorption [11, 20]. Parenteral vitamin supplementation of fat-soluble vitamins and enteral pancreatic enzyme replacement is beneficial for patients with exocrine insufficiency and malabsorption [11]. Pancreatic enzyme supplementation normalizes fat-soluble vitamin, pre-albumin, and ferritin levels in chronic pancreatitis patients without steatorrhea [21]. Enzyme supplementation can be given in the absence of fecal-fat testing in patients with clinical malabsorption (loose, foul-smelling stool; weight loss; muscle wasting; osteopenia) [22]. A 72-h fecal-fat study is the gold standard to detect steatorrhea; however, this test may not be convenient or feasible given the sensitivity and specificity (100 and 95%, respectively) and positive predictive value (90%) of the acid steatocrit random-spot test [23].
Surgical intervention is warranted in our patient presenting with progressive frequency of pancreatitis episodes, severe and recurrent pain, signs of malabsorption, and evidence of pancreatic duct obstruction and dilation. Our patient warrants a duodenal-preserving partial resection of the pancreatic head, longitudinal ductotomy, and lateral pancreaticojejunostomy (Frey Procedure).
Indications for Frey Procedure
Disabling or severe pain with ductal dilation ≥ 7 mm or dilated with multiple strictures
Asymptomatic with ductal dilation ≥ 7 mm or dilated with multiple stricture
Symptomatic inflammatory mass/calcification in pancreatic head causing ductal dilation
Consideration: dilated with multiple strictures has a “chain of lakes” appearance
Intraoperative Technique
Positioning and Preparation
The patient is placed on the operating room table in the supine position. Intravenous access with two large-bore intravenous cannulas or one central venous line is established, along with arterial wave monitoring via a radial arterial line. Appropriate prophylactic antibiotics, urinary catheter, and compression hose-stockings with a sequential compression device are administered or placed prior to incision.
Exposure of the Pancreas
We begin with a bilateral subcostal incision with thorough exploration of the abdomen prior to exposing the pancreas (Fig. 31.2). The greater omentum is elevated off the transverse mesocolon to its origin on the stomach, and the gastrocolic ligament is divided to provide access into the lesser sac exposing the anterior pancreas body and tail (Fig. 31.3a, b). Exposure of the pancreas is challenging in the setting of chronic inflammation, as the posterior wall of the stomach is frequently densely adherent to the pancreas.
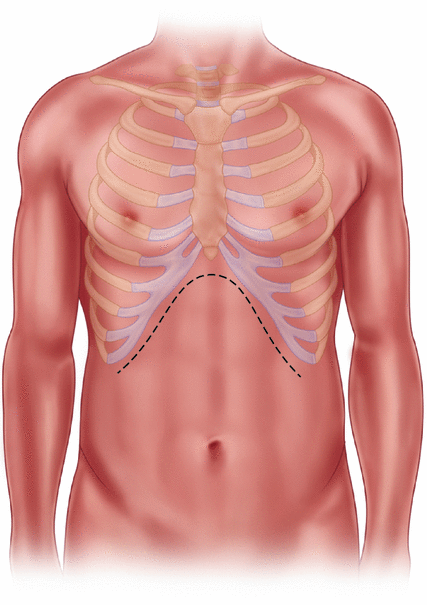
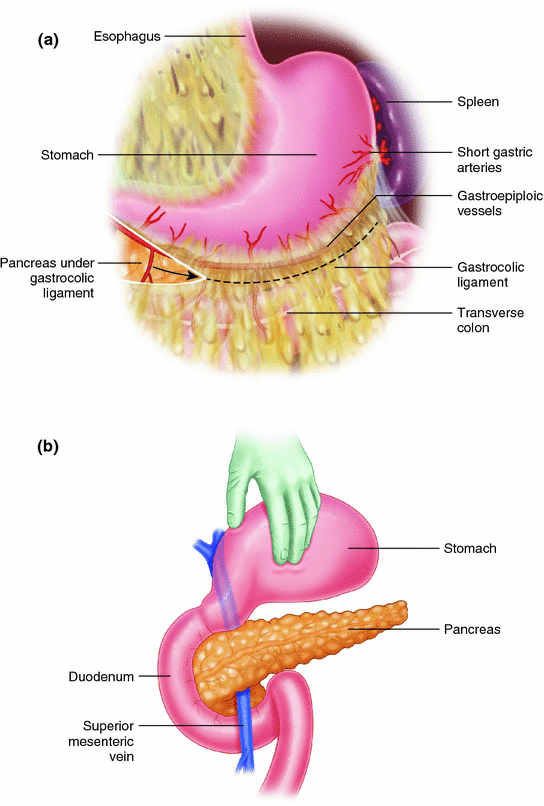

Fig. 31.2
Sub-costal incision or an upper midline incision may be utilized

Fig. 31.3
a Divide the gastrocolic ligament to gain access into the lesser sac. b Reflect the stomach cephalad to expose the anterior pancreas
Longitudinal Pancreatic Ductotomy
First, identify the gastroduodenal artery (GDA) near the head. If the GDA can be palpated, ligate at the superior and inferior border of the pancreas for proximal and distal control, respectively, with interrupted figure-of-eight 4-0 Prolene sutures. If the GDA is not palpable due to extensive fibrosis, carefully dissect until the GDA is encountered, and control with manual compression until ligated. Once dissection approaches the neck, formal control of the GDA is performed superiorly and inferiorly to the pancreas as described. The pancreatic duct is identified within the pancreatic head with a needle and opened using cautery with a high setting (75–85) on fulgurate mode. We insert an appropriately sized probe into the duct upstream toward the tail and cut down along the probe with high cautery. The ductotomy is then directed downstream toward the head to expose the entire main-pancreatic duct up to 1 cm from the papilla of Vater. All pancreatic stones and debris are removed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree