Chronic Nausea and Vomiting
Shalini Dalal
Gayatri Palat
Eduardo Bruera
Chronic nausea and vomiting are common symptoms in patients with advanced cancer and when present are associated with great distress, significantly impacting the quality of life of patients who experience them (1, 2). The reported prevalence of these symptoms varies, depending on patient characteristics and the assessment methods used for diagnosing chronic nausea and vomiting. Fainsinger et al. reported that 71 of 100 patients in a palliative care unit required treatment for nausea in the last week of life (3). Data from the National Hospice Study showed that nausea and vomiting developed in 62% of patients with terminal cancer prevalence rates of 40% in the last 6 weeks of life in women and younger patients reporting higher rates (4). In a prospective study of 1635 patients with cancer referred to a pain clinic, Grond et al. reported a prevalence of 27% for nausea and 20% for vomiting (5).
There is no standardized definition for chronic nausea. For research purposes it is often defined as nausea lasting for more than 4 weeks. In a population of patients with advanced cancer and short life expectancy this definition excludes many patients. For our purposes the presence of nausea for more than 1 week, in the absence of a well-identified, self-limiting cause (e.g., chemotherapy or radiotherapy) will be termed chronic. Chronic nausea has many etiologies, is often multifactorial and requires chronic treatment (3). The various contributing etiologies are presented in Figure 14.1. Unfortunately chronic nausea has not been well studied in the palliative care population and therefore the relative contribution of different causes is not known.
The purpose of this chapter is to review the pathophysiology, causes, assessment, and management of chronic nausea.
Pathophysiology
The pathophysiology of chronic nausea and vomiting is complex with multiple mechanisms of emesis. A number of neural pathways and neurotransmitters are likely to be involved. Much of what we know about nausea and vomiting today is based on research in patients receiving chemotherapy or radiotherapy and in patients with postoperative nausea and vomiting. Chronic nausea in patients with cancer has not been well studied. In this patient population, research is much more difficult because many factors are present that may contribute to the mechanism of emesis. As a result, research on the mechanisms and management of acute chemotherapy or radiotherapy-induced nausea in which there are fewer contributing factors may not apply to the population of patients with chronic symptoms. In addition the importance of different mechanisms are likely to vary between individual patients. The various pathways and centers known to be involved in the emetic pathway are illustrated in Figure 14.1 and described in detail in the following text.
Vomiting Center and Central Pattern Generator
Good evidence exists that the various stimuli affecting nausea and vomiting are relayed to the brain in an area described several decades ago as the vomiting center (VC) (6).
The VC has long been thought to represent the physiologic control center and is located in the lateral reticular formation of the medulla. This center is not a discrete anatomic site, but rather represents a complex array of interrelated neuronal networks coordinated by a central pattern generator (CPG). This site includes the nucleus tractus solitarius (NTS) and the dorsal motor nucleus of the vagus (DMV) (7). The NTS is the site where four major afferent neuronal pathways carrying emetogenic signals converge, as follows:
Peripheral pathways (through the vagus and splanchnic nerves) from the gastrointestinal (GI) tract, visceral capsules, and the parietal serosal surfaces
Neuronal connections from the chemoreceptor trigger zone (CTZ)
Vestibular pathways from labyrinth, in response to vertigo and visuospatial disorientation
Cortical pathways from higher cortical centers, in response to sensory stimuli (pain, sight, smell) and psychogenic stimuli (memory, conditioning, fear)
One or more of these pathways may be involved in a patient experiencing nausea or vomiting. Each of these four areas respond to certain types of emetic stimuli modulated by specific neurotransmitters that bind to specific receptors.
The NTS is viscerotopically organized into subnuclei that subserve diverse functions related to swallowing, gastric sensation, laryngeal and pharyngeal sensation, baroreceptor function, and respiration. Depending on the intensity and duration of received signals from these various sources, the NTS processes this information, and accordingly the DMV puts out an appropriate vasomotor efferent response inducing nausea, retching, or vomiting (8). Although nausea, a subjective symptom, has not been well studied, vomiting comprising of retching and expulsion phases has been researched in experimental
animals. Coordinated combination of events involving neurons controlling the diaphragm, inspiration, blood pressure, heart rate, larynx, pharynx, tongue, esophageal sphincter, and stomach have been found to occur in a sequential manner. Approximately half of the preganglionic motor neurons in the DMV that innervate the lower esophageal sphincter (LES) also innervate the gastric fundus (9). This may have relevance to emesis, as both fundic relaxation and LES relaxation precede emesis. It must be noted that the output neurons that control the muscles involved in emesis are believed to be scattered in the medulla oblongata and therefore there is no discrete site as such. This has supported the concept of the term “central pattern generator” rather than “vomiting center” per se, involving sequential activation of relevant neurons based on the received signals (10). Nausea without vomiting may be due to insufficient stimulation of these neurons. On the other hand, persistent nausea after vomiting may denote persistent stimulation. There is little evidence that antiemetic agents act directly on the CPG.
animals. Coordinated combination of events involving neurons controlling the diaphragm, inspiration, blood pressure, heart rate, larynx, pharynx, tongue, esophageal sphincter, and stomach have been found to occur in a sequential manner. Approximately half of the preganglionic motor neurons in the DMV that innervate the lower esophageal sphincter (LES) also innervate the gastric fundus (9). This may have relevance to emesis, as both fundic relaxation and LES relaxation precede emesis. It must be noted that the output neurons that control the muscles involved in emesis are believed to be scattered in the medulla oblongata and therefore there is no discrete site as such. This has supported the concept of the term “central pattern generator” rather than “vomiting center” per se, involving sequential activation of relevant neurons based on the received signals (10). Nausea without vomiting may be due to insufficient stimulation of these neurons. On the other hand, persistent nausea after vomiting may denote persistent stimulation. There is little evidence that antiemetic agents act directly on the CPG.
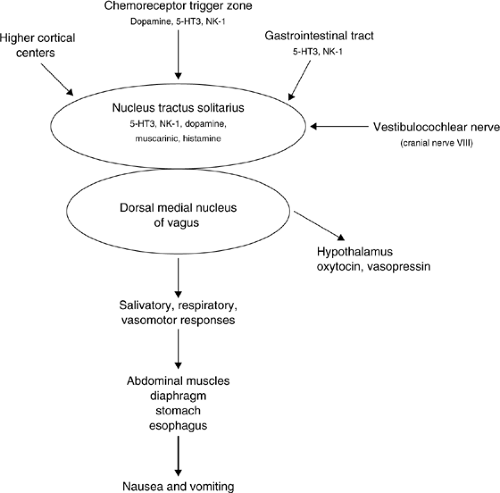 Figure 14.1. Emetic pathways and neurotransmitters. 5-HT3, 5-hydroxytryptamine type 3; NK-1, neurokinin-1 |
Vasopressin and oxytocin levels increase in humans experiencing nausea from both illusory self-motion and a number of emetic-producing treatments (11). Changes in neurohypophyseal hormone release from the hypothalamus are relayed from catecholaminergic groups in the ventrolateral medulla and from the NTS (12). These are identified as part of the central pathway by which afferent abdominal vagal stimulation increases plasma vasopressin and arterial pressure. Rise in vasopressin levels may lead to hyponatremia in patients with chronic nausea.
Chemoreceptor Trigger Zone
The CTZ is situated in the area postrema of the medulla. The CTZ is located functionally outside the blood–brain barrier and therefore samples emetogenic toxins present in cerebrospinal fluid and blood (6). Chemotherapeutic agents, metabolic products (e.g., uremia, hypercalcemia), opioids, and bacterial toxins produce nausea and vomiting by stimulating the CTZ. The CTZ also receives afferent input from peripheral sites (GI tract) through the vagus and splanchnic nerves. The CTZ cannot initiate emesis independently and does so only through stimulation of NTS. A neural pathway connects the two areas.
The predominant neurotransmitters in this area are dopamine (D2), serotonin 5-hydroxytryptamine type 3 (5-HT3), and neurokinin-1 (NK-1) The involvement of D2 has been the basis for the use of drugs with antidopaminergic activity such as butyrophenones (haloperidol), phenothiazines, and metoclopramide. 5-HT3 antagonists and NK-1 receptor antagonists are useful for the treatment of chemotherapy- and radiotherapy-induced nausea.
Cortex
The cortex and other areas of the brain, such as the diencephalon and the limbic system, supply afferents to the VC. Raised intracranial pressure, taste, smell, and anxiety can contribute to and stimulate vomiting. The brain cortex is also involved in emesis associated with thoughts, and visual or olfactory stimuli in patients receiving chemotherapy (13).
Vestibulocochlear Nerve
Motion can trigger nausea and vomiting. Opioids can alter the sensitivity of the vestibular center (14) resulting in nausea associated with movement. Motion stimulates receptors in the
labyrinth. Impulses are then transmitted to the vestibular nucleus and onto the cerebellum, CTZ, and VC. The frequency of nausea and vomiting is comparatively higher in ambulatory patients as compared to those confined to bed. The predominant neurotransmitters involved at this level are acetylcholine and histamine. Anticholinergics and antihistamines are therefore helpful for treatment.
labyrinth. Impulses are then transmitted to the vestibular nucleus and onto the cerebellum, CTZ, and VC. The frequency of nausea and vomiting is comparatively higher in ambulatory patients as compared to those confined to bed. The predominant neurotransmitters involved at this level are acetylcholine and histamine. Anticholinergics and antihistamines are therefore helpful for treatment.
Gastrointestinal Tract
Stimulation of mechanoreceptors or chemoreceptors in the gut can cause nausea and vomiting. 5-HT3, D2, acetylcholine, histamine, and substance P (SP) are important neurotransmitters involved in stimulating these receptors. SP binds to NK-1 receptors in the gut (and directly in the vomit center in the brain). GI obstruction, gastric stasis, metastatic disease, bacterial toxins, drugs, chemotherapeutic agents, and irradiation can cause emesis in this way. Abdominal vagal afferents that detect intestinal luminal contents and gastric tone terminate in the NTS (15). In addition, stimulation of the glossopharyngeal or vagus nerves in the pharynx by sputum, candida, or mucosal lesions can cause nausea. Acetylcholine in addition to being a neurotransmitter also increases gut motility and gut secretion.
Etiology
Chronic nausea in patients with advanced cancer is often multifactorial. Figure 14.2 summarizes the common causes of chronic nausea in this patient population. In many patients the underlying cause or causes are difficult to determine.
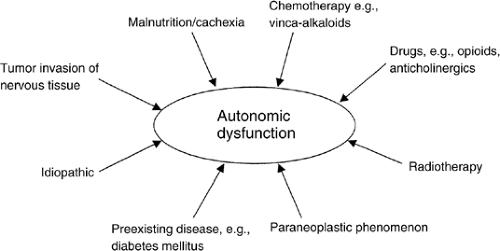
Autonomic Failure
In some patients with cancer, chronic nausea is associated with the anorexia cachexia syndrome. In these patients autonomic failure is thought to be the most likely cause of chronic nausea (16). Autonomic failure causes gastroparesis with resulting anorexia, nausea, and early satiety. In addition, other effects on the GI tract include diarrhea and constipation. Autonomic failure also has cardiovascular manifestations such as postural hypotension, syncope, and fixed heart rate.
Autonomic failure was originally described in patients with diabetes mellitus, neurologic disorders, and chronic renal disease (17). It has also been described in patients with advanced cancer. Kris et al. (18) reported delayed gastric emptying in ten patients with cancer who complained of chronic nausea and vomiting. Bruera et al. (19) looked at the incidence of cardiovascular autonomic insufficiency in 43 patients with advanced breast cancer and in 20 healthy controls matched for age and sex. Tests for autonomic failure were performed, and 52% of tests were abnormal in the patient group versus 7% in the control group. Autonomic failure in the group of patients with cancer was more common in those with poor performance status and malnutrition. In another study which included five patients with advanced cancer complaining of unexplained chronic nausea and anorexia, 16 of 23 tests for autonomic function were abnormal, compared to none of 25 tests performed in the control group of five healthy adults (20). None of the five patients had clinical or laboratory evidence of disseminated disease to the abdomen or liver. All had normal endoscopy and barium meals with no evidence of mucosal injury. All five patients had severe gastroparesis (mean emptying time 192 ± 28 minutes) as compared to five controlled (mean emptying time 66 ± 5 minutes) when assessed with a gastric emptying scan. The differences between patients and controls were statistically significant; it was concluded that gastroparesis was the cause of chronic nausea and anorexia in these patients. There have been several other cases reported in the literature of autonomic dysfunction associated with lung and pancreatic cancers (21, 22, 23).
The cause of autonomic dysfunction in patients with advanced cancer remains unclear and appears to be multifactorial. Malnutrition itself has been suggested as a cause of autonomic neuropathy (16). Jewish physicians in the Warsaw ghetto during World War II found that their patients who were starving were not able to increase their blood pressure with effort and had a constant tachycardia with no change on standing up (24). Features of autonomic dysfunction have also been described in patients with anorexia nervosa. Studies on animals have suggested that fasting suppresses the activity of the sympathetic nervous system (25). There are isolated case reports suggesting autonomic dysfunction as a paraneoplastic manifestation of advanced cancer (22, 26, 27). Park et al. (26) reported a patient with bronchial carcinoma who showed postural hypotension and abnormal tests for autonomic dysfunction that disappeared after irradiation of the tumor. In a
second report, a patient with small cell carcinoma of the lung developed intestinal obstruction and abnormal tests for autonomic dysfunction. An autopsy demonstrated degeneration of autonomic nerves without tumor involvement in the area. Antineuronal antibodies have been identified in patients with small cell lung carcinoma who demonstrated neurologic signs consistent with autonomic neuropathy (28).
second report, a patient with small cell carcinoma of the lung developed intestinal obstruction and abnormal tests for autonomic dysfunction. An autopsy demonstrated degeneration of autonomic nerves without tumor involvement in the area. Antineuronal antibodies have been identified in patients with small cell lung carcinoma who demonstrated neurologic signs consistent with autonomic neuropathy (28).
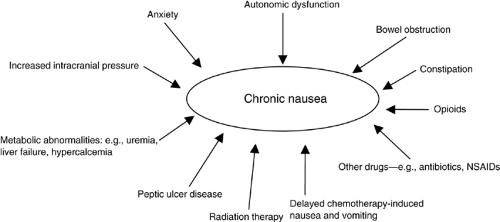 Figure 14.2. Causes of chronic nausea in patients with advanced cancer. NSAIDs, nonsteroidal anti-inflammatory drugs |
Another possible etiologic factor in autonomic neuropathy in this patient population is direct tumor involvement (29). Cardiovascular autonomic neuropathy has been noted after the administration of chemotherapeutic agents such as vinca alkaloids (30). Drugs such as opioids, anticholinergics, antidepressants, and vasodilators may also have adverse effects on the autonomic nervous system. Many of these drugs are used in the management of cancer-related symptoms. Radiation damage to the autonomic ganglia is also a potential factor. Human immunodeficiency virus (HIV) infection has also been noted to produce autonomic insufficiency (31).
Autonomic dysfunction is a frequent feature in patients with advanced cancer. It should be suspected in patients with unexplained tachycardia, poor performance status, chronic nausea, and malnutrition. Figure 14.3 summarizes the possible causes of autonomic dysfunction in patients with advanced cancer.
Drugs
Opioids are one of the most common causes of chronic nausea in patients with cancer. It is estimated that 6–12% of these patients may experience nausea, with some studies reporting its occurrence as high as 29% (32, 33). It may often be difficult to differentiate the cause of opioid-induced nausea due to various comorbidities, conditions, and concomitant drugs, thereby skewing an accurate estimate of its true frequency (34). Patients may often associate nausea with a recent opioid analgesic, creating potential barriers to effective pain management in the form of anticipatory nausea, anxiety, or adherence problems. Opioid analgesia can cause nausea and vomiting in patients after initiation or increase in dose. This usually responds well to antiemetic medication and disappears spontaneously within the first 3 or 4 days of treatment. Some patients, particularly those receiving high doses of opioids, experience chronic and severe nausea (2).
Opioids cause chronic nausea by a number of mechanisms, including stimulation of the CTZ, gastroparesis, constipation, and by increasing sensitivity of the vestibular center. The CTZ being located outside the blood–brain barrier samples emetogenic drugs present in the circulation including opioids, which act on specific nerve cells or opioid receptors. The presence of opioid receptors in the CTZ in the area postrema is consistent with the observation of morphine and enkephalin-induced vomiting in the dog after local or systemic administration and the abolishment of this response after ablation of the area postrema (35). The CTZ also accepts several medullary neurons, which may be responsible for inhibiting the action potential of these specific nerve cells, thereby keeping the neurons of the CTZ from firing more readily. Neurons originating from the medulla are hypothesized to be enkephalinergic. It is theorized that displacing them by opioids or opioid antagonists may predispose patients to nausea by, in effect, removing inhibitory input into the CTZ. Chronic nausea has been associated with accumulation of active morphine metabolites such as morphine-6-glucuronide. Accumulation of morphine metabolites is more likely with higher doses of opioids but can occur even with lower doses, especially in patients with renal insufficiency (36).
By an unknown mechanism, the vestibular apparatus is directly stimulated by most opioids. This may add to the already decreased threshold for nausea they cause at the CTZ. Distention of the gut, decreased GI emptying time, and constipation may stimulate mechanoreceptors. Stimulation of visceral mechanoreceptors and chemoreceptors is most commonly responsible for nausea and emesis in terminally ill patients receiving opioid drugs. Opioids also decrease GI emptying time, which may cause constipation or fecal impaction. Although not as well defined as the other neuronal inputs, the cortex has direct input into the VC through several types of neuroreceptors. A patient may remember unpleasant feelings of nausea associated with past opioid therapy. When presented with the sight, smell, or even anticipation of taking an opioid again, a strong nausea reflex may result.
Many other drugs can cause nausea. Nonsteroidal anti-inflammatory drugs, antibiotics, and iron supplements can cause nausea irritation of the GI tract. Antibiotics may also cause nausea by a direct effect on the CTZ. Psychoactive medications including tricyclic antidepressants, selective 5-HT3 reuptake inhibitors, and phenothiazines can cause nausea.
Constipation
Constipation is common in patients with advanced cancer. Severe constipation can cause nausea and vomiting, abdominal pain, distension, urinary retention, and cognitive failure (37). There are many factors in patients with advanced cancer that predispose to the development of constipation, including opioid analgesics, immobility, poor oral intake and dehydration, autonomic failure, and other medications. Constipation
is often underdiagnosed in this patient population and should be suspected as a cause of nausea in all patients with advanced cancer (38).
is often underdiagnosed in this patient population and should be suspected as a cause of nausea in all patients with advanced cancer (38).
Bowel Obstruction
Bowel obstruction is a less common but important cause of nausea and vomiting. It has been reported to occur in 3% of terminally ill patients with cancer, and was found to be present in 15 of 100 consecutive patients admitted to a tertiary palliative care unit (39). Although acute complete bowel obstruction is easy to diagnose, most patients with advanced cancer tend to present with a less clear picture of slow progression from partial to complete bowel obstruction. Some patients have only intermittent obstructive symptoms. These episodes of obstruction may be accompanied by nausea and vomiting.
Assessment
Because the causes of chronic nausea and vomiting are often multifactorial, the assessment in a given patient should be multidimensional, with awareness that these symptoms are dynamic processes and frequently change in intensity. There are a number of effective systems for assessing the intensity of nausea, such as visual analog scales, numerical scales, and verbal descriptors, but there is no “gold standard” for nausea assessment. As nausea is a subjective symptom, its expression varies from patient to patient and will depend on the individual patient’s perception and other factors such as psychosocial issues. From a practical point of view this occasionally leads to a lack of correlation between the observed expression of nausea and the presumed pathophysiology of the underlying condition. It is also important to note that the term nausea may mean different things to different individuals and is used by some patients to describe other symptoms, including abdominal discomfort, pain, distension, or early satiety.
Once the intensity of nausea has been assessed, it is important to assess the symptom in the context of other symptoms such as pain, appetite, fatigue, depression, and anxiety. This multidimensional assessment allows formulation of a therapeutic strategy. An example of a validated multidimensional assessment tool is the Edmonton Symptom Assessment System (40, 41) The system is based on visual analog scales that assess pain, nausea, anxiety, depression, appetite, shortness of breath, and sense of well-being, among others. Tools such as this can be used to document the intensity of nausea initially, as a baseline assessment, and then on a regular basis, giving the examiner a sense of the therapeutic effect of the management. Such tools allow for a more reproducible assessment from the point of view of research and quality control.
A detailed history and physical examination is essential. It is important to clarify that the patient’s expression of nausea does not describe a different symptom such as reflux. Intensity, frequency, exacerbating and relieving factors, onset, and duration of nausea should be documented. If there is coexistent vomiting, the nature of the vomiting should also be documented as the quantity of the vomitus can give a clue to the etiology. Large-volume emesis may indicate gastric outflow obstruction whereas small volume emesis may indicate gastric stasis. The extent to which the emesis interferes with oral intake should be noted, as large and frequent volume vomiting puts the patient at risk of rapid dehydration. A history of syncopal episodes or early satiety should alert the physician of the possibility of autonomic insufficiency.
The etiology of nausea may be related to the underlying cancer diagnosis. It is important to obtain details of tumor involvement and spread, as well as treatment history. In patients with intra-abdominal involvement, nausea with or without vomiting is often seen due to liver metastasis, mechanical obstruction of bowel by tumor, or peritoneal carcinomatosis. The stomach and duodenum can be compressed, causing the “squashed-stomach syndrome.” Nausea may be present secondary to primary or metastatic brain involvement by tumor, or leptomeningeal disease. Radiation therapy to the spine or abdomen may be followed by nausea and vomiting.
A detailed medication history is essential. The use of drugs capable of causing nausea, such as opioids, anticholinergics, nonsteroidal anti-inflammatory drugs, and antibiotics, should be specifically noted. Delayed chemotherapy-induced nausea and vomiting (CINV) may be present and refers to symptoms that occur 24 hours after chemotherapy administration and which may last for as many as 6 to 7 days. Cisplatin has an approximate 65-90% likelihood of causing delayed emesis in the absence of antiemetic prophylaxis (42). Patients with HIV may have nausea, which is a side effect of all the drugs of the highly active antiretroviral therapy (HAART) regimen. Patients should be questioned about recent use of steroids because their abrupt inadvertent discontinuation could lead to addisonian crisis presenting with nausea, vomiting, abdominal pain, and hypotension. Emotional experiences, history of anxiety disorder should be explored. Other medical conditions, such as peptic ulcer disease and diabetes, should also be noted. Autonomic failure should be suspected in patients with diabetic neuropathy or chronic renal failure.
Constipation, as already discussed (See the section Constipation), is a common complication in patients with advanced cancer and may cause or aggravate nausea. The frequency of bowel movements; feeling of abdominal distension; rectal soreness; oozing of stool; change in the amount of gases or stool passed recently; laxative use, type, and dose; and date of last bowel movement should be obtained. Unfortunately, clinical impression is likely to be quite unreliable and investigations, such as a plain abdominal x-ray, should be undertaken to help in the diagnosis. The use of a radiologic constipation score may be necessary for adequate diagnosis in some patients, particularly those with cognitive failure. On a plain abdominal x-ray the abdomen is divided into four quadrants. Each quadrant is assessed for constipation: score 0 = no stool; 1 = stool occupying <50% of the lumen; 2 = stool occupying >50% of the lumen; 3 = stool completely occupying the whole lumen of the colon. The total score of all quadrants is calculated and will range from 0 to 12. A score of 7 or greater is suggestive of severe constipation (38).
Physical examination may be helpful in identifying possible underlying causes. Table 14.1 summarizes possible findings on physical examination in patients with chronic nausea and vomiting.
Physical examination may be helpful in identifying possible underlying causes. Table 14.1 summarizes possible findings on physical examination in patients with chronic nausea and vomiting.
Table 14.1 Possible Findings on Physical Examination in Patients With Chronic Nausea and Vomiting | |||
|---|---|---|---|
|
Investigations to exclude renal impairment, hepatic failure, and other metabolic abnormalities such as hypercalcemia, hypokalemia, and hyponatremia should be undertaken. A computed tomography scan of the brain may be indicated where brain metastases are suspected. Abdominal x-rays may be useful in assessing nausea. A supine x-ray may indicate the presence of stool and fecal impaction. Erect or decubitus views may show air and fluid levels in the bowel, which is typical of bowel obstruction. Sophisticated investigations, such as gastric emptying scans, are probably not justified for most patients.
Management
Appropriate management of chronic nausea and vomiting depends on a detailed assessment. General supportive measures should be instituted in all patients. In addition specific interventions should be undertaken as appropriate. Due to the lack of well-designed studies there is no convincing data as to the best pharmacologic strategy for treatment of nausea and vomiting and current management is on the basis of expert opinion rather than evidence (43).
Many palliative care specialists are now favoring a mechanistic approach to nausea management, where the initial choice of medication is based on the likely mechanism, and neuropharmacology of the emetic pathway. Response rates of 80–90% have been reported with this approach (44, 45). An alternative approach of empiric treatment has been recommended by some, and in studies found to be highly effective (46, 47, 48, 49). There have been no head to head comparisons of these approaches.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree