Allogeneic hematopoietic stem cell transplantation (HSCT) revolutionized the outlook for many patients with chronic myeloid leukemia (CML) in the 1980s. The introduction of the tyrosine kinase inhibitors (TKIs) nearly 15 years ago displaced HSCT as the first-line treatment for most CML patients. However, in the twenty-first century HSCT remains a viable treatment option for many patients with CML. This review focuses on the role of HSCT for CML in the TKI era, paying particular attention to patient selection and transplant outcome.
Key points
- •
Tyrosine kinase inhibitors have replaced hematopoietic stem cell transplantation (HSCT) in the first-line treatment of chronic myeloid leukemia (CML) in its chronic phase.
- •
HSCT remains a viable treatment option.
- •
HSCT remains the standard of care for patients with accelerated or blast-phase CML.
Introduction
Chronic myeloid leukemia (CML) is clonal stem cell disorder driven by the BCR-ABL1 fusion gene resulting from a reciprocal translocation between chromosomes 9 and 22 (the Philadelphia chromosome). It is a triphasic disease, with most patients presenting in chronic phase (CP) characterized by hepatosplenomegaly, thrombocytosis, and leukocytosis of mature granulocytes and their precursors. Until relatively recently, the natural disease history dictated that patients remained in CP for several years before progressing to the accelerated (AP) and blast (BP) phases, which were inevitably fatal. The first reports of CML date back to the mid-nineteenth century, and early treatment strategies included arsenic, splenic radiation, busulfan, and hydroxycarbamide. Although these approaches were effective in symptom control, they did little to alter the natural progression of the disease to BP. During the 1980s, allogeneic hematopoietic stem cell transplantation (HSCT) became the first, and remains the only treatment capable of consistently eradicating the malignant clonal stem cell population. For several decades allogeneic HSCT remained the gold standard treatment for younger patients with suitable human leukocyte antigen (HLA)-identical donors. The identification of the BCR-ABL1 fusion gene in 1983, and the understanding that this encoded a dysregulated tyrosine kinase capable of autophosphorylation and activation of downstream proteins, led to the development of the tyrosine kinase inhibitors (TKIs). Today, for most patients the ultimate goal of leukemic eradication and cure with HSCT has been superseded by a target of deep molecular remission and long-term disease control with an oral agent. Undoubtedly, the TKIs have now replaced HSCT as the first-line treatment of CP CML. Nevertheless, allogeneic HSCT remains a valuable treatment option for CML, and this review focuses on its current role.
Introduction
Chronic myeloid leukemia (CML) is clonal stem cell disorder driven by the BCR-ABL1 fusion gene resulting from a reciprocal translocation between chromosomes 9 and 22 (the Philadelphia chromosome). It is a triphasic disease, with most patients presenting in chronic phase (CP) characterized by hepatosplenomegaly, thrombocytosis, and leukocytosis of mature granulocytes and their precursors. Until relatively recently, the natural disease history dictated that patients remained in CP for several years before progressing to the accelerated (AP) and blast (BP) phases, which were inevitably fatal. The first reports of CML date back to the mid-nineteenth century, and early treatment strategies included arsenic, splenic radiation, busulfan, and hydroxycarbamide. Although these approaches were effective in symptom control, they did little to alter the natural progression of the disease to BP. During the 1980s, allogeneic hematopoietic stem cell transplantation (HSCT) became the first, and remains the only treatment capable of consistently eradicating the malignant clonal stem cell population. For several decades allogeneic HSCT remained the gold standard treatment for younger patients with suitable human leukocyte antigen (HLA)-identical donors. The identification of the BCR-ABL1 fusion gene in 1983, and the understanding that this encoded a dysregulated tyrosine kinase capable of autophosphorylation and activation of downstream proteins, led to the development of the tyrosine kinase inhibitors (TKIs). Today, for most patients the ultimate goal of leukemic eradication and cure with HSCT has been superseded by a target of deep molecular remission and long-term disease control with an oral agent. Undoubtedly, the TKIs have now replaced HSCT as the first-line treatment of CP CML. Nevertheless, allogeneic HSCT remains a valuable treatment option for CML, and this review focuses on its current role.
Indications for transplantation
Chronic-Phase Chronic Myeloid Leukemia
Three of the 5 TKIs currently available are licensed for first-line treatment in CML, namely imatinib, dasatinib, and nilotinib. The goal of therapy is to induce deep and durable responses using an agent that can be tolerated in the long term, because for most patients treatment will be lifelong.
The response to TKI therapy is the most important prognostic factor, and is assessed on 3 levels; hematologic, cytogenetic, and molecular. The European Leukemia Network (ELN) have established criteria defining complete hematologic response, complete cytogenetic response (CCyR), and major molecular response (MMR), and require that these responses be achieved by certain time points. Recently updated guidelines use these milestones to stratify response into categories of optimal, warning, and failure. Furthermore, this recent version has incorporated the finding by several groups that an inability to achieve a 10-fold reduction in tumor load by 3 months, as indicated by a real-time, quantitative, reverse transcriptase–polymerase chain reaction (RQ-PCR) result of less than 10%, is associated with a relatively poor long-term survival.
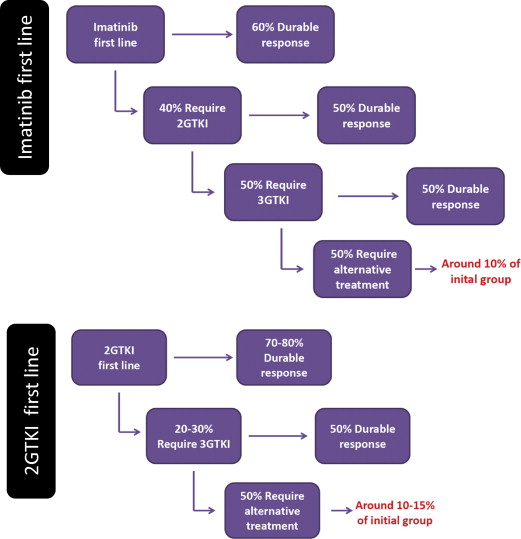
Imatinib is capable of inducing CCyR in the most patients and MMR in many, both of which are associated with prolonged long-term survival. It is this level of efficacy that has led to its adoption as first-line therapy. However, despite its success, by 8 years more than 40% of patients will discontinue imatinib because of intolerance and/or lack or loss of response. The second-generation TKIs (2GTKIs) dasatinib, nilotinib, and bosutinib have now all been used as front-line therapy. Trial data show that their higher potency in inhibiting BCR-ABL1 translates to superiority in achieving cytogenetic and molecular responses. Two large prospective, randomized clinical trials comparing one or other of these agents with imatinib demonstrated prolonged durable remissions, with nilotinib achieving MMR at 3 years in 70% to 73% of patients compared with 53% with imatinib, and dasatinib achieving MMR in 69% compared with 55% with imatinib. Although the data for bosutinib are less mature, similar superiority over imatinib has been demonstrated, with MMR rates of 41% at 12 months compared with 27% with imatinib. In both trials of nilotinib and dasatinib, failure to achieve an RQ-PCR of less than 10% at 3 months was associated with an increased risk of treatment failure. Moreover, in both studies approximately 30% of patients had discontinued their front-line therapy at 3 years, indicating ongoing problems with longer-term efficacy and tolerability.
The 2 main reasons necessitating TKI cessation are intolerance and resistance. Toxicities such as nausea and diarrhea, muscle cramps and arthralgia, edema, and rashes can be common, particularly at the initiation of therapy. Later, patients taking imatinib complain of chronic debilitating fatigue. Typically these side effects are mild and can often be managed with temporary treatment interruption or dose reduction; however, for some they are severe and intolerable, and require cessation of treatment with the particular drug. Dasatinib and nilotinib have some more serious toxicities, only rarely (or never) observed with imatinib, including pleural effusion and pulmonary arterial hypertension on dasatinib and hyperglycemia, abnormal liver function tests, and vascular thrombotic events on nilotinib.
Drug resistance can occur for several reasons. It may be primary, whereby from the outset there is no or little response to the drug, or it can be secondary, developing after a period of initial response. The mechanisms of resistance are beyond the remit of this review, although broadly there are 2 main categories: resistance with an associated kinase domain (KD) mutation and resistance without an identifiable KD mutation. In most cases leukemic cells containing KD mutations are thought to be present at the time of diagnosis and can be observed (or selected) only after nonmutated cells have been killed by the TKI. These mutations (single-nucleotide polymorphisms) may render the protein resistant to 1 or more TKI. Although knowledge of the site of a KD mutation may guide the choice of 2GTKI, many patients will be resistant to their therapy without evidence of a KD.
Patients who fail front-line treatment with imatinib may respond to a change to a 2GTKI. Second-line dasatinib is associated with a 6-year progression-free survival (PFS) of 40% to 50%, with overall survival (OS) on the order of 70%. Of note, in the largest phase II trial of 670 patients, only 28% of those treated with dasatinib remained on the study drug 6 years later. Similar patterns of response are seen in patients treated with nilotinib as second-line therapy following prior imatinib. The largest phase II trial of nilotinib in imatinib-resistant or intolerant patients showed that CCyR was achieved in 45% of patients with OS and PFS at 48 months of 78% and 57%, respectively. Again at 48 months, importantly 70% of patients had discontinued treatment, the most common reason being disease progression. The data from these 2 large studies show that although 2GTKI can induce a durable remission in many patients, most of those who require a 2GTKI will subsequently require an alternative therapy, one of which might be HSCT. Just as 3-month BCR-ABL1 burden is predictive of long-term survival in front-line TKI, the same is true of second-line therapy. Those patients who fail to achieve BCR-ABL1 of less than 10% after 3 months of second-line therapy have a significantly poorer long-term outlook, and the predictive power of such an early assessment could be helpful in the identification of potential HSCT candidates.
Early data on the use of a third-generation TKI, ponatinib, the only drug to be active against the T315I mutation, suggest that approximately 40% of patients resistant or intolerant to multiple TKIs will achieve CCyR, with 27% achieving MMR : while both CCyR and MMR are predictors of prolonged survival, mature data are awaited. In patients with the T315I mutation responses are slightly better, with CCyR and MMR rates of 66% and 56%, respectively; however, about half of these patients will still require alternative treatment. In this circumstance allogeneic HSCT is the only option for long-term survival.
In summary, approximately 10% to 15% of patients presenting in CP will ultimately fail to achieve a durable remission with any TKI ( Fig. 1 ). For these patients, allogeneic HSCT may offer a strategy to achieve long-term remission.
Advanced-Phase Chronic Myeloid Leukemia
The outcome for patients who present in either AP or BP, or who develop AP or BP while on treatment, is extremely poor. Frequently these patients carry multiple additional nonrandom chromosomal abnormalities, and those previously exposed to a TKI may also harbor KD mutations. In contrast to CP, TKIs have had less impact on the outcome of AP, and very little impact on the outcome of BP disease.
In BP, the median OS has only risen from 3 to 4 months pre-TKI to 7 to 11 months in the post-TKI era. Although often these patients will initially respond to TKI therapy, almost invariably their response is partial and transient, thereby generating a window of opportunity for definitive treatment such as HSCT. HSCT in the second CP offers the best prospect of long-term survival for these patients, with less than 10% achieving long-term survival if transplanted in frank BP. Although TKI treatment may never achieve CCyR and MMR in patients presenting with BP, imatinib can briefly induce hematologic remission in 50% to 70%. For those progressing to BP during treatment with a TKI, cytoreductive chemotherapy in combination with a 2GTKI seems to offer the best prospect of returning the patient to CP, permitting consideration of HSCT.
For AP patients the impact of TKIs is more positive. Imatinib is capable of achieving CCyR in around 60% of patients. 2GTKIs show promise in achieving better responses (MMR 76%), although with only small numbers of patients described to date. At present, durable response rates remain too low to confidently withhold HSCT in eligible patients. Jiang and colleagues suggested a risk-stratification algorithm capable of identifying low-risk patients in whom HSCT conferred no advantage over imatinib (6-year OS 81% vs 100%), but these patients constituted less than 1 in 3 AP patients. The outcome for high-risk patients who did not receive HSCT was dismal (5-year OS 18% vs 100%).
Preparation for transplant
The decision to proceed to allogeneic HSCT in the TKI era must take into account several factors. As already discussed, disease-specific factors may influence the decision to transplant, such as phase of disease and probability of response to 2GTKI in imatinib-resistant patients. The second major factor to consider is fitness for transplant. Patient-specific factors such as age and comorbidities are known to predict mortality following HSCT, and the risk of transplant must be carefully balanced against the risks of disease progression.
Assessment of Transplant Risk
In the late 1990s the European Group for Blood and Marrow Transplant (EBMT) developed a risk-scoring system for patients with CML based on a data set of more than 3000 patients, at a time when CML was the commonest indication for allogeneic HSCT. This system is based on 5 variables ( Table 1 ) and is a powerful predictor of both survival and transplant-related mortality (TRM), with predicted 5-year OS ranging from 72% to 20% in the low- to high-risk groups, respectively. These data were accumulated from patients who had not been exposed to TKI. Of interest, a recent reanalysis of the EBMT database using patients transplanted after exposure to TKI shows that the score remains prognostic. The EBMT score has been independently validated by the Center for International Blood and Marrow Transplant Research (CIBMTR), and is also valuable in the assessment of patients undergoing a second allogeneic HSCT in CML. In addition to the EBMT score, recent data have shown that comorbidities at the time of transplant significantly affect transplant outcome. The hematopoietic cell transplantation comorbidity index (HCT-CI) has been used to predict non-relapse mortality and OS in many hematologic malignancies. More recently, its use has been validated as an independent predictor of TRM and OS in CML patients undergoing allogeneic HSCT and, importantly, many of the patients used for its validation had been pretreated with TKIs, justifying its use in current practice. This study also identified the presence of an elevated C-reactive protein at the time of admission for transplant, as an independent predictor of increased TRM and reduced OS.
| Risk Factor | Category | Score |
|---|---|---|
| Donor type | HLA-identical sibling | 0 |
| Matched unrelated donor | 1 | |
| Disease stage | First chronic phase | 0 |
| Accelerated phase | 1 | |
| Blast crisis | 2 | |
| Age of recipient (y) | <20 | 0 |
| 20–40 | 1 | |
| >40 | 2 | |
| Sex combination | All except: | 0 |
| Male recipient/female donor | 1 | |
| Time from diagnosis to transplant (mo) | <12 | 0 |
| >12 | 1 |
| Risk Score | Probability of Outcome at 5 y (%) | ||
|---|---|---|---|
| LFS | OS | TRM | |
| 0 | 60 | 72 | 20 |
| 1 | 60 | 70 | 23 |
| 2 | 47 | 62 | 31 |
| 3 | 37 | 48 | 46 |
| 4 | 35 | 40 | 51 |
| 5 | 19 | 18 | 71 |
| 6 | 16 | 22 | 73 |
Timing of Transplant
In current practice, TKIs will almost always be the first line of treatment in a newly diagnosed CML patient. However, tools now exist for identifying patients who are likely to fare poorly with first-, second-, and third-generation TKIs. Although prediction of a poor outcome in these patients should not preclude a trial of alternative drugs, it should alert the clinician to the prospect that allogeneic HSCT may offer the best prospect of long-term remission, prompting early discussion about HSCT with the patient in addition to early identification of potential donors. For this reason, the authors would advocate HLA typing and donor identification in patients listed in Table 2 .
| Triggers for Initiation of an HLA Donor Search (sibling’s HLA typing, followed by unrelated donor search) | |
| Chronic phase |
|
| Accelerated or blast phase | All patients deemed fit for allogeneic HSCT |
In patients with AP or BC, there is no doubt that allogeneic HSCT offers the best prospect of long-term survival, although timing of transplant is crucial. The best possible response must be sought before HSCT, and the time from achieving best response to proceeding to HSCT minimized. Ideally patients should be transplanted as soon as practical after achieving second (or higher) CP.
Allogeneic transplant outcome
More than 3 decades have passed since the first reports of bone marrow transplant for CML. At the time, the eradication of the Philadelphia positive clonal cells by myeloablation and successful syngeneic donor transplant was revolutionary, and marked the start of a new chapter in the evolution of CML management. In the following 20 years pioneering work resulted in a surge of HSCT for CML, which led to both cure from the disease for thousands of patients and the establishment of the principles on which much of the current transplant practice is based. In the subsequent 15 years following the introduction of the TKIs, there has been an equally steep decline in the number of transplants. However, the legacy of data means that following careful patient selection, CML is one of the most well understood and evidence-based indications for HSCT.
Early transplant outcomes, despite offering cure for many, were hampered by high TRM. Between 1980 and 1990 more than 2600 patients underwent allogeneic HSCT in Europe for CML, and the TRM approached 40% with 20-year OS rates of 40%, 20%, and 10% for CP, AP, and BP, respectively. More recent data show that improvements in transplant procedures and supportive care have significantly improved outcomes and have decreased TRM. Data from the CIBMTR in 2008 documented survivals of 79% and 72% at 1 and 2 years, respectively, for HSCT undertaken between 1999 and 2004, and the most recently transplanted patients within the German CML study IV showed further improvements, with 3-year OS in excess of 90%.
For patients in AP or BP a favorable TRM, combined with the dismal long-term outlook with alternative strategies, dictates HSCT as the best long-term strategy. For CP patients, however, one is faced with several more intricate questions regarding who and when to transplant. (1) Do transplant outcome data from the pre-imatinib era translate to patients who have tried and failed TKI therapy? (2) Does prolonging the time to transplant by trying multiple TKIs worsen transplant outcome? (3) Should HSCT remain a first-line option for any patients? Some of the questions are addressed by the recent literature on transplant outcome in CML, which is summarized in Table 3 .