John C. Byrd and Joseph M. Flynn • Chronic lymphocytic leukemia (CLL) is the most prevalent type of adult leukemia and is defined by a distinctive immunophenotype of CD19+, CD20+, CD5+, CD23+, and surface immunoglobulin (sIg)-positive cells. • The environmental or genetic cause of CLL in most patients is not known, although 8% to 10% of patients have a first-degree relative with this diagnosis. • CLL lacks a single driving mutation that defines the disease, although autonomous B-cell receptor signaling appears to be important; the role of stem cells remains controversial. • Classic staging for CLL at diagnosis is not helpful in most patients for risk stratification, but new prognostic factors such as immunoglobulin variable heavy chain (IGHV1) mutational status, interphase cytogenetics, β2-microglobulin, and thymidine kinase activity have improved staging of this disease. • Common disease-related complications associated with CLL include immune deficiency with associated infections, autoimmune complications, secondary cancers, and Richter transformation. • CLL is treated only when it becomes symptomatic, based on earlier studies showing no survival advantage to early intervention. This same approach is followed for all patients irrespective of genetic risk. • The current standard treatment for young and older patients with CLL is chemotherapy combined with an anti-CD20 monoclonal antibody. Although this prolongs survival in CLL, it does not cure the disease. • Reduced-intensity allogeneic stem cell transplant is curative in a subset of CLL patients but still carries with it treatment-related morbidity and mortality. • B-cell receptor antagonists offer great promise as a new targeted therapy for CLL. B-cell chronic lymphocytic leukemia (CLL) is one of the most commonly occurring leukemias in the Western Hemisphere, accounting for approximately one third of all leukemias. CLL was initially described by Dameshek as an accumulative disorder of abnormal immunologically incompetent lymphocytes.1 Our understanding of the biology of CLL has changed dramatically as new basic science investigation has brought forth many new discoveries showing that the disease not only involves disrupted apoptosis but also has a strong dependence on microenvironment and proliferation. Similarly, the clinical evolution of CLL is now recognized to involve a precursor syndrome called monoclonal B-cell lymphocytosis of uncertain significance that has the potential to progress to full-blown CLL at a frequency of 1% to 2% per year. The cause of CLL in most cases is not recognized, although there is a strong genetic predisposition to this disease in select families. Despite this high genetic predisposition, no common single-gene mutation has been associated with CLL as compared with other solid tumors such as breast and neuroendocrine tumors. CLL is no longer defined by morphology alone but rather immunophenotypically based on co-expression of traditional mature B-cell markers (CD19, CD20, dim surface immunoglobulin) with the pan–T-cell marker CD5. Despite much improved understanding in the biology of CLL, treatment of this disease is still not undertaken until symptoms arise, based on no evidence of improvement in survival with early treatment of asymptomatic individuals. Although for many decades treatment of symptomatic CLL was also not associated with improvement in survival, the introduction of chemoimmunotherapy has finally demonstrated improvement in this important end point. CLL is still not cured with this type of treatment, and immune suppression promoted both by progression of the disease and therapies used leads to many of the complications patients with this disease experience. The long natural history of CLL, complications arising from both the disease and treatment, and psychosocial issues occurring at different phases provide challenges to both the hematologist and primary care physician involved in the care of patients with this diagnosis. CLL is one of the most common types of leukemia in the Western Hemisphere. The Surveillance, Epidemiology and End Results Program (SEER) estimated that 16,060 patients (9,490 men and 6,570 women) would be diagnosed with CLL and 4,580 would die as a consequence of this disease in 2012 (http://seer.cancer.gov/). The median age at diagnosis for CLL was 72 years during 2005 through 2009 according to the SEER database. Unlike other types of leukemia, CLL is extremely rare in individuals younger than the age of 20, making it exclusively a cancer of adults. The proportion of patients diagnosed with CLL increases with age. The frequency of diagnosis among CLL patients is 1.8% in persons 44 and younger; 9.0% between ages 45 and 54; 20.9% between ages 55 and 64; 26.5% between ages 65 and 74; 27.8% between ages 75 and 84; and 14.0% in those older than age 85. The age-adjusted incidence rate was 4.2 per 100,000 per year; this translates into a risk of approximately 1 in 202 patients being diagnosed with CLL during their lifetime. In general, CLL is more common in men, with a 2 : 1 frequency. Race and ethnicity contribute to the frequency of CLL, with the disease being most common in white (6.1/100,000) followed by black (4.3/100,000), American Indian (2.5/100,000), Hispanic (2.4/100,000), and Asian (1.3/100,000) men. The survival rate following diagnosis in CLL is 78.8% at 5 years, which explains the estimated prevalence of 95,123 patients currently living with CLL in the United States. Five-year relative estimated survival rates by race and sex are 77.6% for white men, 80.9% for white women, 64.0% for black men, and 69.1% for black women. The worse outcome for black patients was confirmed in another recently published paper.2 Similar to that for other types of leukemia, the risk of dying of disease-specific CLL causes increases proportionately with increasing age. Another analysis of the SEER database comparing outcome of elderly patients with CLL to age- and sex-matched healthy controls demonstrated that CLL has the greatest impact on survival in the most elderly group of patients. However, even for patients diagnosed with CLL before the age of 50, Montserrat and colleagues demonstrated that the median expected life span is only 12.3 years, compared with 31.2 years in age-matched controls.3 Thus, CLL is a significant health problem that can affect all ages of patients. The varied frequency of CLL among different ethnic and racial backgrounds provides the opportunity to study the influence of environment on development of the disease. CLL is rare among Asians and Pacific Islanders, and this persists even in immigrants from these areas who have migrated to the Western Hemisphere.4,5 This implicates a possible genetic predisposition to the development of CLL. The relationship of environmental factors such as exposure to benzene and other chemicals to the development of CLL is not clearly defined. Select epidemiology studies have identified a higher risk of CLL among farmers and others with exposure to pesticides,6 but these findings have not been consistent.7 However, CLL is recognized as a service-connected illness among Vietnam War veterans who were exposed to Agent Orange (http://www.publichealth.va.gov/exposures/agentorange/). For patients in the theatre of the Vietnam conflict, it is important to identify this factor because additional compensation from the U.S. Department of Veterans Affairs is possible. Occupational or environmental exposure to radiation does not appear to predispose patients to a higher risk of developing CLL as compared with other types of leukemia.8 This lack of increased risk for developing CLL after excessive radiation exposure was observed in both survivors of the atomic bomb at Hiroshima9,10 and also inhabitants of the Chernobyl nuclear reactor accident.11 In contrast, the frequency of acute myeloid leukemia, acute lymphoblastic leukemia, and chronic myeloid leukemia was higher after exposure to radiation in these settings. Recently, a precursor to CLL termed monoclonal B-cell lymphocytosis (MBL) has been identified12 that has many similarities to monoclonal gammopathy of uncertain significance (MGUS) in multiple myeloma. The frequency of MBL in the general population is 3%, with increasing frequency of diagnosis based on age and also familial history of disease.13 Of patients with MBL, 1% to 2% will progress each year to meet the criteria of CLL (i.e., 5 × 109/L of malignant B cells in the blood), and risk for progression is best predicted by the absolute lymphocyte count.14,15 Although this condition until recently has been viewed as having little impact on outcome, several studies have identified an increased risk of infectious morbidity as compared with the age-matched control population without MBL.16 At this point, MBL should be viewed as not a malignant diagnosis because only a small subset of patients progress to a pathological disease. However, as with MGUS patients, these individuals do warrant serial follow-up with blood tests and physical examination to ensure signs and symptoms of CLL are not developing. The blurred definition between MBL and early-stage CLL has been exacerbated recently by changes in the definition of CLL from requiring 5 × 109/L lymphocytes to 5 × 109/L B lymphocytes.17 This change downstages a subset of early-stage CLL patients to MBL, and long term could influence survival estimates of CLL by removing patients with the most favorable prognosis.18 Differences between MBL, CLL, and small lymphocytic leukemia (SLL) are summarized in Table 102-1. Table 102-1 Characteristics of Monoclonal B-Cell Lymphocytosis (MBL), Chronic Lymphocytic Leukemia (CLL), and Small Lymphocytic Leukemia (SLL) The knowledge of CLL biology and genetics has expanded tremendously during the past decade. This in part has occurred fortuitously from CLL patients with a large volume of readily available primary tumor cells this situation has facilitated easy application of new technologies to study gene expression profiling, miR profiling, and RNA/DNA whole-genome sequencing. These new technologies have allowed researchers to sort out important questions debated for many years in editorials, review papers, and book chapters related to the cell of origin of CLL. Based on the classic CD5, CD19, CD20, and dim surface immunoglobulin phenotype of CLL, for many years the cell of origin was assumed to be that of a B1 peritoneal B lymphocyte. This finding was challenged by application of gene expression profiling of normal B cells at different points in development and also distinct B-cell malignancies.19,20 Although the normal counterpart cell of origin of transformed CLL still remains controversial, it appears to be most closely related to a memory B cell.19 Within a distinct gene expression pattern for all CLL is the ability to differentiate genes that segregate the natural history of CLL from indolent to aggressive (see later discussion of IGHV1 mutational status). In addition, expansion of other technologies has expanded further the knowledge and risk stratification of CLL, including whole-exon and whole-genome sequencing projects in CLL that have identified genes such as NOTCH1 and SF3B121 that have a relatively high (10% to 20%) frequency of mutation in CLL and clearly have clinical relevance with respect to risk stratification and also treatment. The biology and genetics covered within this chapter focus predominantly on areas relevant to the clinician who cares for patients with CLL. For many years, the treatment of cancer has been characterized by attempts at eliminating macroscopic tumor cells that are the visible phenotype of the disease. Detailed study of normal hematopoiesis has demonstrated a very small number of normal stem cells that when isolated from the bone marrow could repopulate and establish normal human marrow function in irradiated, immune-deficient mice.22 These observations eventually led to successful application of both allogeneic and also autologous bone marrow transplantation as a curative treatment option for many patients with hematologic malignancies. Derived from this work was the hypothesis that leukemia (and potentially other cancers) was also derived from a small proportion of “leukemia” stem cells that proliferate and differentiate to produce more mature blast cells that ultimately represent the visible phenotype of the disease. The implications of such a finding are great, because the properties of stem cells are quite different from the more mature blast cells to which therapies were adapted. Research over the past 2 decades in acute leukemia, multiple myeloma, and many different solid tumors has provided significant support for this hypothesis. In contrast to other types of leukemia, for many years it was believed that CLL was not derived from a clonal stem cell, based on the inability of the leukemia cells to engraft and recapitulate the disease when tumor cells are inoculated or transplanted into immunocompromised mice. However, a recent sentinel paper demonstrated that bone marrow CD34+ hematopoietic stem cells from CLL patients have a higher number of cells with B-lymphoid progenitor phenotype and develop clonal B cells characteristic of CLL when engrafted into irradiated, immunocompromised mice.23 More notably, these cells demonstrated the ability to also be re-engrafted into irradiated, immunocompromised mice and again develop findings consistent with CLL, demonstrating true stem cell properties of these cells. In contrast to the CD34+ cells from the CLL bone marrow, this group also showed that lymphocytes lacking CD34 never demonstrated long-term engraftment in the mice. As compared with both normal CD34+ stem cells and more differentiated B-cell progenitor cells, these CLL stem cells had different gene expression, including absent NOTCH1 and increased GATA2 expression. Notably, the CLL cells developing in these mice after the first and successive engraftments did not have the genetic features of the patient’s CLL, suggesting that an additional transforming event or events in the clonal CLL cells may occur. Specifically, neither the common genetic translocations seen in the patient, that is, del(13q14), nor IGHV1 mutational status was the same for the CLL found in engrafted mice. These findings suggest overall that CLL patients have lymphoid primed stem cells and via acquisition of different genetic abnormalities, CLL ultimately develops. Although this paper describing CLL stem cells is quite provocative,23 it lacks validation by other groups working in the field. The findings, if true, will likely focus significant research attention on differentiating properties of these CLL stem cells from normal CD34+ stem cells to allow novel treatment approaches. Additionally, studies pursuing why Ighv1 status of tumors in the mouse uniformly is highly mutated will be pursued because this suggests some intrinsic feature of either a second genetic hit or an environmental influence is driving development of this high-risk phenotype CLL. Based on the CD5+ status of CLL cells and the presence of surface igD (sigD) on CLL cells, this disease for many years was considered to be derived from a naïve B cell without somatic hypermutation that typically occurs in the germinal center. The process of somatic mutation is generally assessed by examining amino acid changes occurring in the first and second complementarity determining regions (CDR1 and CDR2). Early studies in the 1990s of small numbers of CLL patients demonstrated the sequence of immunoglobulin variable heavy (VH) chain genes was unmutated (germline), thereby providing further support that the cell of origin of CLL was a naïve B-cell. These studies were later challenged by several small reports and then by a more comprehensive study in 1994 by Schroeder and Dighiero, who identified approximately one half of the reported cases of CLL had VH genes with less than 98% sequence similarity to germline.24 Given the extent of extensive polymorphisms in this region, the 98% number for similarity to germline was established, although subsequent studies have challenged this as too high. The hypothesis from this thought-provoking paper by Schroeder and Dighiero was that CLL might be a heterogeneous disorder that could be differentiated on the basis of IGHV1 mutational status.24 It was 5 years before two separate research groups demonstrated that the IGHV1 gene had undergone somatic mutation, indicating that the patient’s CLL arose after this point in B-cell maturation, in 60% of CLL patients at diagnosis.25,26 In contrast, approximately 40% of patients had germline sequence appearance of the IGHV1 region mimicking a pre-germinal B-cell phenotype. From each of these papers came the very interesting observation that IGHV1 mutational status was highly segregated with disease outcome. Patients with IGHV1-mutated disease who had early-stage disease had a very long natural history with only a subset progressing to the point of requiring therapy, whereas those with IGHV1-nonmutated disease tended to have a much more aggressive natural history with virtually all requiring therapy and also having a shortened survival. Furthermore, many of the other high-risk genetic features (see later discussion) including del(17p13.1), del(11q22.3), 53 mutations, complex karyotype, shortened telomeres, and clonal evolution were identified to be associated with IGHV1-nonmutated status.27–34 At the time IGHV1 mutational status was identified, the technology available to widely export this finding to clinical practice was lacking. One of these papers suggested CD38 could serve as a surrogate of IGHV1 mutational status.26 Subsequent studies showed that like IGHV1 mutational status, CD38 was an unfavorable prognostic factor but changed during the course of the disease and only had approximately a 70% concordance with IGHV1 mutational status.27,28,35–39 This led to the active investigation of both surrogate markers of IGHV1 mutational status and also the biology behind it. The scientific impact of identification of different clinical history among IGHV1-mutated and nonmutated CLL prompted great interest in CLL after the sentinel discovery in 1999. Several other investigators used new techniques of gene expression profiling to pursue identification of surrogate markers that could predict the presence or absence of IGHV1 mutational status and also elucidate new genes that might contribute to the biology. Rosenwald and Staudt were the first to identify a gene expression profile of CLL that identified it as one disease with a common signature.20 However, select gene expression distinguished IGHV1-nonmutated from IGHV1-mutated disease. Most notably was expression of the 70-kDa zeta-chain–associated protein (ZAP70). Other genes such as lipoprotein lipase were also noted to be differentially expressed and have been actively pursued by others.20,40,41 However, great focus in the field has focused on ZAP70 contribution to the biology of CLL. ZAP70 is a tyrosine kinase of the Syk family that acts centrally in T-cell receptor signaling. At the time of this study there was no known expression of ZAP70 protein in normal or transformed B cells. This work and others that followed confirmed that the majority of IGHV1-nonmutated CLL cells have ZAP70 expression and demonstrate evidence of SYK activation and other essential B-cell receptor (BCR) downstream activation signals after ligation of surface IgM (sIgM) that is related to overexpression of this protein.42,43 Of interest, several studies suggested that the kinase portion of ZAP70 is not required for enhanced BCR signaling because transfection of a kinase dead ZAP70 construct into CLL cells lacking this protein showed enhanced BCR signaling with IgM ligation.44,45 In contrast, cells from virtually all IGHV1-mutated patients lack significant ZAP70 expression and do not signal after ligation by sIgM, but can often weakly signal through other alternative BCRs. Subsequent studies examining ZAP70 for activating mutations have been unrevealing.46 Additionally, attempts to introduce ZAP70 into mouse models with B-cell CLL predisposition have not accelerated the phenotype of the disease. These studies have left questions as to the impact of ZAP70 as a driver in the pathogenesis of aggressive CLL. CLL has distinct BCR signaling as compared with normal B cells that is characterized by low-level IgM expression, variable response to antigen stimulation, and tonic activation of anti-apoptotic signaling pathways. CLL cells by mRNA expression share many features with antigen-activated mature B cells, suggesting a role for activation of BCR signaling in the disease pathogenesis. A recent publication validated the importance of BCR signaling but showed that it is antigen-independent cell-autonomous signaling.47 The importance of BCR signaling was further substantiated in a tissue-based comparison demonstrating enhanced upregulation of the BCR pathway–related genes by microarray in bone marrow and lymph nodes of all CLL patients as compared with blood irrespective of ZAP70 expression or IGHV1 mutational status.48 Thus, enhanced BCR gene expression is present in all CLL patients in nodal and lymph node sites. Additionally, patients with ZAP70-positive disease have higher BCR responsiveness to IgM stimulation in vitro compared with ZAP70-negative disease. Dysregulation of the BCR signaling pathway in CLL is characterized by constitutively active phosphorylation of certain kinases and variable response to IgM stimulation. The best evidence of this lies in the tyrosine kinases Lyn and Syk, both having been shown to be upregulated in CLL. Activity of these kinases has also been shown to be amplified in primary CLL cells. Specifically, in vitro kinase assays indicate that Lyn is constitutively active in CLL.49 Baseline tyrosine phosphorylation of Syk as well is higher in CLL cells than normal B cells,50 whereas response to antigen stimulation via the BCR signaling pathway is variable.51 Although protein expression of the catalytic 110δ subunit, the predominant phosphatidylinositol-3-kinase (PI3K) subunit in hematopoietic cells,52 is comparable between normal B cells and CLL cells, PI3K has been shown to be constitutively active in CLL by in vitro kinase assay.53,54 Additionally, inhibition of PI3K by the pan-PI3K inhibitor LY29400253 and the PI3Kδ inhibitor GS110154 both promote CLL cell apoptosis in a caspase-dependent manner. Inhibition of PI3K by CAL-101 or LY294002 inhibits Akt activation, decreases myeloid cell leukemia sequence 1 (BCL2-related) (MCL1) expression,53,54 and inhibits protein expression of the B-cell CLL/lymphoma 2 (BCL2) family member BCL2-associated X protein (BAX) and the anti-apoptotic X-linked inhibitor of apoptosis protein (XIAP).53 The Btk enzyme essential to BCR signaling has also been shown to have increased expression in CLL and evidence of autophosphorylation in a subset of patients, providing additional evidence for activation of this pathway.55 Btk activation leads to pro-survival signals through its effects on PI3K, PLCγ2, and nuclear factor κB (NF-κB). Inhibition of Btk by the kinase inhibitor ibrutinib induces apoptosis in a caspase-dependent manner and inhibits both phosphorylation of Btk after IgM ligation as well as downstream targets of Btk activation, including Erk, NF-κB, and Akt.55 Additionally, ibrutinib inhibits proliferation by CpG or microenvironment stimulation.55,56 Collectively, these studies provide evidence of the importance of BCR signaling CLL, which has led to introduction of new therapeutics for this disease. Since its initial description and early characterization, CLL has long been considered a disease of slow accumulation of tumor cells, resulting from disrupted or defective apoptosis. Multiple studies have demonstrated that CLL cells overexpress several anti-apoptotic proteins, including BCL2, MCL1, BCL2-antagonist/killer 1 (BAK), and XIAP and have diminished expression of compensatory pro-apoptotic proteins such as BAX.57 The balance of expression of these anti-apoptotic proteins in CLL cells in vivo has been shown to be quite volatile once the tumor cells are removed from their microenvironment, where apoptosis is often rapidly noted.58,59 Studies by multiple groups have demonstrated that CLL cells have in vivo constitutive activation of several anti-apoptotic transcription factors, including NF-κB, nuclear factor of activated T cells (NFAT), and signal transducer and activator of transcription 3 (STAT3) that can influence one or more of the anti-apoptotic proteins that promote survival in vivo.60–63 The source of activation of these different transcription factors is not completely defined but may in part be due to autocrine and paracrine networks involving tumor necrosis factor ligand superfamily member 13b (TNFSF13B); acidic (leucine-rich) nuclear phosphoprotein 32 family, member B (APRIL); vascular endothelial growth factor (VEGF); interleukin (IL)-4; and TNF receptor superfamily member 5 (CD40) and provide an explanation of why many of the downstream survival anti-apoptotic genes decrease when out of the body.59,64 CLL cells are also maintained through contact with stromal cells (bone marrow and dendritic) and nurse similar cells through a complex interface of adhesion molecules and stromal survival factors such as stromal cell–derived factor (SDF-1).65–68 Much of the advances in the understanding of the biology of acute leukemia have come from studying repetitively occurring cytogenetic abnormalities. Such detailed study of the genetics of CLL has been hindered by the inability to effectively induce proliferation of tumor cells for standard metaphase cytogenetic analysis and the poor response of CLL cells to B-cell mitogens. Nonetheless, several historic cytogenetic studies identified a variety of deletions, including del(11q22.3), del(17p13.1), del(13q14), and del(6q21-q23), as well as trisomy 12, as common abnormalities in CLL.69,70 The frequency of these abnormalities has been further refined through the use of interphase cytogenetic analysis, which does not require isolation of dividing cells. These studies have demonstrated that del(13q14) is by far the most common cytogenetic abnormality in CLL, followed by trisomy 12, del(11q22.3), del(17p13.1), and del(6q22.3).71 Stimulation studies with CpG oligonucleotides plus IL-4 or CD40 confirmed the prevalence of these abnormalities and also identified unbalanced translocations not generally observed with traditional metaphase cytogenetics.72,73 The prognostic significance of these unbalanced translocations appears to be important, and a study by the CLL Research Consortium demonstrated that these could be produced reproducibly from the same patient within different cytogenetic laboratories, which lowers the concern that this is an artificial finding of in vitro tumor stimulation.74 Similarly, complexity of karyotype, as already appreciated in acute leukemia, appears to be a poor prognostic factor in CLL. These genetic findings have been further refined with single-nucleotide polymorphism (SNP) or comparative genomic hybridization arrays from which a negative impact of complexity75 has been established. Additionally, new, yet undescribed deletions have been noted for chromosome 15 at the MGA locus76 that will require further study to determine their impact on biology and relevance to prognosis of CLL patients. A perplexing finding that needs to be reconciled is why CLL stem cells lack these common cytogenetic abnormalities. Current research would suggest that many of these are secondary events as part of the leukemia progression. Application of new technologies moving forward will likely facilitate better characterization of recurring genetic abnormalities in CLL. The presence of recurrent deletions in CLL suggests the possibility of unique tumor suppressor genes in these different regions that has led to extensive study for more than 2 decades. In particular, attention to coding genes within the 13q14 region failed to identify a viable tumor suppressor gene candidate for many years. However, in 2002, Croce and colleagues identified miR15 and miR16, two noncoding microRNAs, in the deleted region of 13q14.77 Noncoding RNAs range in size from 21 to 25 nucleotides and represent a newly recognized class of gene products whose function is to silence genes through binding to the 3′-untranslated region of specific genes to inhibit translation. When near-compatible hybridization of the noncoding RNA exists, RNA transcription can also be antagonized. This same group later showed that miR16 regulates expression of BCL2, which is overexpressed in CLL and other B-cell lymphoproliferative disorders.78 Multiple different studies have associated specific miR expression with rapid disease progression, fludarabine resistance, and poor prognosis. In addition, miR34a has been directly related to the adverse outcome associated with TP53 dysfunction.79,80 At the time of this writing, several reports are coming forth about the role of miRs in cell-to-cell communication. Further study of miRs in CLL is underway to elucidate their full role in the pathogenesis and progression of this disease. In addition, other conserved larger noncoding RNAs with different small interfering RNA (siRNA) and epigenetic silencing roles have been identified to have a significant role in CLL. Until the advent of whole-exon and whole-genomic sequencing, CLL was not typically associated with recurring mutations early in the pathogenesis of the disease. Probably most characterized are p53 mutations, which occur in 3% to 5% of patients at diagnosis, often in conjunction with deletion of the alternative allele (at 17p13.1 loci) that is associated with rapid disease progression and poor survival.83–83 With treatment and subsequent relapse, the frequency of p53 mutations continues to increase proportionately and is most common in patients with Richter transformation.86–86 Another factor upstream of p53 that has also been shown to be recurrently mutated in 10% to 20% of patients is ataxia telangiectasia mutated (ATM).89–89 The ATM gene lies in the region of minimal deletion of del(11q22.3) that is commonly seen in CLL. ATM mutations are not exclusively observed in patients who have del(11q22.3), although loss of function of both alleles has been associated with diminished DNA damage response.90 Impact of ATM mutation on outcome in CLL has been reported in some,91,92 but not all93 studies. The major impact in CLL biology related to recurring mutations has come with the advent of availability of whole-exon and whole-genomic DNA sequencing. During the past year, two papers have been published in which the authors identified unique properties of CLL cells compared with studies done in solid tumors relative to mutation frequency and also have identified several novel candidate genes with recurrent mutations that warrant further study. Puente and Campo were the first to report four individual patients (two IGHV1-mutated vs. two IGHV1-nonmutated) who had whole-genome sequencing.94 This study identified approximately 1000 somatic mutations per tumor in nonrepetitive regions similar to that observed in acute leukemia but much lower than that observed in lung cancer or malignant melanoma. The authors also detected a marked differences in the mutation pattern between CLL samples with IGHV1-mutated patients having a higher proportion of A>C/T>G mutations than cases with IGHV1-nonmutated disease. The authors hypothesized that differences between CLL subtypes might reflect the molecular mechanisms implicated in the IGHV1 mutational subtype. Specifically, for the IGHV1-mutated patients, an error-prone polymerase η during somatic hypermutation could contribute to the high frequency of A > T to C > G transversions. Extending this, the authors identified 46 somatic mutations that caused changes in the protein-coding sequences of 45 genes in the four patients studied. None of these nucleotide substitutions had been previously linked to CLL pathogenesis, and only one had been reported in a CLL patient (NOTCH1). This group validated 26 of these 46 genes that were expressed at an mRNA level in 169 CLL patients and identified four genes (NOTCH1, MYD88, XPO1, and KLHL6) that were present in at least one additional patient.94 Surprisingly, NOTCH1 mutations were present in approximately 12% of CLL samples at a site that deletes a proteolytic site in the carboxy (C) terminus, thus resulting in increased protein stability; furthermore, gene induction as determined by microarray analysis was consistent with elevated NOTCH pathway activity. Patients with NOTCH1 mutations were shown to have more aggressive disease, as measured by stage and also overall survival (OS). The findings by this group relative to incidence of NOTCH1 have been confirmed by several other groups, including high association of NOTCH1 mutations with treatment-refractory CLL.95–99 The authors also identified MYD88 as a recurrent mutation in IGHV1-mutated CLL that had functional significance based on enhanced Toll-like receptor signaling. The functions of less common XPO1 and KLH6 mutations were not explored. A second paper by this same group examining whole-exon sequencing21 together with another group led by Wu100 identified SF3B1, encoding a subunit of the spliceosomal U2 small nuclear ribonucleoprotein (snRNP) as another recurring gene in CLL. Similar to the first paper characterizing NOTCH1, a phenotype of disordered splicing in CLL cells bearing SF3B1 mutations was identified. SF3B1 mutations were noted to be more frequently associated with del(11q22.3) and IGHV1-nonmutated status and had negative impact on OS.100 SF3B1 mutations similar to the mutation seen in CLL have been found in other types of leukemia and myelodysplastic syndrome. The biology behind how these mutations might contribute to the development or progression of CLL at this time is unknown. Similar to the gene expression profiling studies that identified ZAP70 expression as an important potential contributor to the biology of CLL more than a decade ago, whole-genome and whole-exon sequencing are now bringing forth new candidate genes that before these studies had little predicted relationship to CLL. These studies to date however provide evidence that there is likely not to be a common driving mutation such as seen with BRAF V600 mutations in hairy cell leukemia101 and MYD88 mutations in Waldenström macroglobulinemia.102 Effective immune surveillance with protection from infection and malignancy requires a coordinated effort between the innate and adaptive components of the immune system. A central driving feature of the pathogenesis of CLL is early immune deficiency that facilitates tumor growth and expansion while evading classic immune surveillance by both the innate and adaptive immune system. The best evidence of the potential immune suppressive features of CLL cells comes from studies in which allogeneic T cells combined with CLL cells were unable to generate mixed lymphocyte reaction and cytotoxicity toward these tumor cells.103 Studies examining the function of the innate and adaptive immune system have documented early defects at diagnosis that tend to progress with expansion of the disease. For instance, studies have shown that the absolute number of T cells and natural killer (NK) cells and the presence of hypogammaglobulinemia in CLL patients at diagnosis have been shown to predict OS.104,105 With progression of disease from diagnosis to time of treatment, several studies have noted both expansion of suppressive T-regulatory cells106,107 and also change in CD4 helper cells from a T-helper (Th) type 1 (Th1) anti-tumor cytokine, anti-tumor type profile (tumor necrosis factor-α [TNF-α] and interferon-γ [IFN-γ]) to predominantly a Th2 anti-inflammatory cytokine profile (IL-4, IL-10) that facilitates CLL survival in the microenvironment.108 The immunosuppressive effects observed in CLL lie predominantly with the CLL clone, as recently demonstrated in very elegant work by the Gribben laboratory in humans and also representative mouse models of CLL.109 The Gribben group showed CLL cells express antigens such as CD200, CD270, CD274, and CD276 that functionally diminish the ability of both allogeneic and also autologous T cells to activate.110 In addition to the loss of direct anti-tumor cytotoxicity by CLL T cells, this defect also leads to much of the infectious morbidity associated with this disease. The importance of reversing the immune deficiency in CLL represents goals of therapy and of diminishing long-term morbidity caused by infections and secondary malignancy in this disease. It has been appreciated that for decades that CLL cells have a great tendency to undergo spontaneous apoptosis when removed from the body, similar to many other lymphoid tumors. Concomitant with this observation, several groups identified that CLL cells when grown on stromal cells65,66 or together with chemokines59,111 or integrin components112,113 relevant to CLL increased greatly their survival in culture.65,66 Furthermore, elegant studies by several groups showed that co-culture on bone marrow, mesenchymal, or nurselike cells enhanced CLL cell proliferation and also upregulated select anti-apoptotic genes such as MCL1, BCL2, and A1. Upregulation of these anti-apoptotic genes (and loss in culture) is one reason CLL cells in protected sites such as bone marrow and nodes have enhanced resistance to cytotoxic agents. In addition to enhancing anti-apoptotic genes, these same microenvironments can also promote CLL cells to secrete chemokines that can attract T cells that ultimately contribute further to CLL survival and growth in CLL proliferation centers.114,115 This direct and indirect cross-talk between CLL cells and the microenvironment is an area of significant investigation in CLL and, in part, forms the basis for many of the new targeted kinase inhibitors and also therapeutics such as stromal-derived factor 1 (SDF1)/chemokine (C-X-C motif) receptor 4 (CXCR4) antagonists that are currently under evaluation. The diagnosis of CLL for many years has been made based on morphologic criteria of mature lymphocytes on the blood smear with an abundance of smudge cells. Smudge cells are an artificial remnant of the blood smear preparation, but in several series their number has been associated with adverse outcome. Specifically, patients with fewer smudge cells tend to have a more aggressive clinical course.118–118 Preparation of the smear from ethylenediaminetetraacetic acid (EDTA) versus EDTA and bovine serum albumin preparation can greatly influence the number of these cells as well based on their membrane features.119 Despite morphologic similarity, many diseases can mimic CLL in both appearance and clinical presentation. With the advent of distinct genetic markers that distinguish outcome and also new, more effective targeted therapies for CLL and other diseases, determining the correct diagnosis is of great importance. Immunophenotyping (flow cytometry) is the key test utilized to make the diagnosis of CLL and is an essential test at diagnosis. CLL cells have a relatively consistent immunophenotype, which differentiates CLL from mantle cell lymphoma, hairy cell leukemia, follicular center cell lymphoma, splenic lymphoma with villous lymphocytes, and other indolent B-cell malignancies.120,121 Specifically, CLL cells express a variety of B-cell markers, including dim surface immunoglobulin (sIg), CD19, dim CD20, and CD23, as well as the pan–T-cell marker CD5. Kappa or lambda light-chain restriction is always present, establishing the presence of a clonal B-cell population, although sIg expression may be so dim that light-chain restriction may be difficult to determine. In contrast, presence of CD10, FMC7, or CD79b (all typically absent on CLL cells) or bright expression of CD11c, CD20, or CD25 (all typically dim on CLL cells) suggests an alternative low-grade B-cell lymphoproliferative malignancy or a variant that often is typical of trisomy 12. Expression of CD5 without CD23 suggests mantle cell lymphoma, and fluorescence in situ hybridization (FISH) for t(11;14) or immunohistochemical staining for CCND1 overexpression should be performed to exclude mantle cell lymphoma. Repeat immunophenotyping is generally done only to confirm tumor antigen expression if antibody (i.e., rituximab or ofatumumab)-based therapy is to be undertaken or the clinical history suggests that either CLL transformation to a more aggressive phenotype (i.e., prolymphocytic leukemia or large cell lymphoma [Richter transformation]) has occurred. In this setting the immunophenotype may remain the same or have changed, often with loss of CD5 or increased intensity of CD20, FMC7, or CD79b antibodies. If morphologic appearance of prolymphocytes or large lymphoid cells in blood, bone marrow, or lymph nodes is present, flow cytometry changes are not required to make this diagnosis. A bone marrow biopsy and aspirate are typically not required to make a diagnosis of CLL. It is important to understand that to make the diagnosis of CLL there must be at least 5 × 109/µL immunophenotypically confirmed clonal B lymphocytes. A bone marrow at diagnosis is not required to establish the diagnosis by the new International Workshop on Chronic Lymphocytic Leukemia (IWCLL) 2008 recommendations. Our practice is only to do a bone marrow study at diagnosis if there is cytopenia or some other finding at the time of presentation that raises suspicion for Richter transformation. Similarly, a confirmatory lymph node biopsy is not required at the diagnosis of CLL unless an alternative diagnosis is suspected based on the history and findings of physical examination. Staging for any type of cancer is performed at diagnosis to predict how the disease will impact outcome. In general, staging is not applied longitudinally through the course of the disease. The two most common staging criteria for CLL are the Rai122 and the Binet123 staging systems. Both were developed utilizing clinical parameters of disease and were the cornerstone of prognostic decision making until more recent molecular markers became available. The Rai classification was published in 1975122 and was the first disease-specific staging methodology for CLL (Table 102-2). This incorporated several clinical and laboratory manifestations of disease and correlated with median survival of patients. The Rai staging system includes five stages, 0 to 4, that are assigned based on the following: stage 0, presence of lymphocytosis; stage I, lymphocytosis with enlarged lymph nodes; stage II, lymphocytosis with organomegaly and or lymph node enlargement; stage III, lymphocytosis with anemia (hemoglobin < 11 g/dL); and stage IV, lymphocytosis with thrombocytopenia (platelets < 100 × 109/L). Poorer median survival was directly associated with increasing stage and ranged from 150 months for stage 0 to 19 months for stage IV. In studying a 129 French cohort of patients with CLL, Binet and colleagues reported a slightly modified staging schema in 1977 that has since been consolidated to three stages (Table 102-3). As with the Rai staging, Binet staging is based on clinical manifestations of disease and was associated with survivability and prognosis in their population. The modified Binet staging123 contains three groups: stage A is lymphocytosis with less than three nodal areas involved, stage B is lymphocytosis with three or more nodal areas involved without anemia or thrombocytopenia, and stage C is lymphocytosis with or without lymph node or splenomegaly but anemia (hemoglobin < 10 g/dL) or thrombocytopenia (platelets < 100 × 1012/L). Outcomes between these two staging systems are relatively similar, particularly for patients with more advanced stage disease who are cytopenic and in whom survival with traditional therapies is quite short. In North America, the Rai staging system is widely used, whereas in Europe the Binet staging system is used. Both of these staging systems are not very effective at risk-stratifying patients with early-stage disease. As a consequence of this, recent advances in staging systems have begun incorporating cytogenetic and molecular features of the CLL tumor cells as well as to better refine outcome, particularly among patients with early-stage disease.124,125 Table 102-2 *Data from Rai KR, Sawitsky A, Cronkite EP, et al: Clinical staging of chronic lymphocytic leukemia. Blood 1975;46:219–234. Table 102-3 *Data from Binet J, Auquier A, Dighiero G, et al: A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 1981;148:198–206. Although lymphadenopathy is a common clinical feature of CLL and is incorporated into both major clinical staging systems, computed tomography (CT) was not available at the time these systems were devised. A common question arises as to the value of CT as part of the initial staging of newly diagnosed CLL. Several retrospective studies have recently examined whether the incorporation of CT in initial staging affects the ability to predict disease progression at diagnosis. One study examined abdominal CT scans in 140 serially seen patients with stage 0 CLL at a single institution and identified enlarged lymph nodes in 38 of them (27%).126 Patients with enlarged nodes had other high-risk features, including increased bone marrow infiltration, short lymphocyte doubling time, higher lymphocyte count, and increased ZAP70 expression. Patients with abdominal lymph nodes had a shorter treatment-free survival (3.5 years vs. not reached). This study lacked inclusion of all standard biomarkers used for predicting outcome and was too small for a multivariate analysis to definitively show the benefit of CT in this population. Other studies have confirmed127 or not shown the definitive benefit of CT over routine physical examination for decisions relative to treatment and response.128,129 At the present time, the current IWCLL 2008 treatment guidelines and also the National Comprehensive Cancer Network (NCCN) guidelines do not recommend the need for CT evaluation of lymph nodes in the absence of symptoms referable to the abdomen.17,130 Similarly, although CT has been studied the most, positron emission tomography (PET) should also be briefly mentioned. Given the low proliferative index and metabolic activity in CLL cells, PET is generally not helpful for staging this disease.131 However, PET may be useful in detecting Richter transformation. In a single-institution study of 37 patients, PET identified 10 of 11 patients who had documented Richter transformation by tissue biopsy.132 However, 9 patients had false-positive scans. Thus, PET appears to be sensitive for Richter transformation, with a high negative predictive value, but specificity is poor. We generally use these studies to identify patients who warrant biopsy for Richter transformation and to localize where to perform it. Thymidine kinase is an enzyme involved in the salvage pathway of DNA synthesis and correlates with proliferative activity of tumor cells. Elevated thymidine kinase activity (TKA) has been observed to be predictive of early progression in a subgroup of untreated patients with smoldering CLL.135–135 The β2-microglobulin (β2M) is an extracellular protein component of the human leukocyte antigen class I complex. β2M has been shown to have significant prognostic relevance in lymphoma and multiple myeloma and correlates with disease burden in CLL. Hallek and colleagues examined 113 CLL and immunocytoma patients for β2M levels and TKA and demonstrated that elevated TKA and β2M both were independent predictors of shortened progression-free survival (PFS).133 Keating and colleagues confirmed the prognostic value of β2M in 622 CLL patients, reporting that an elevated level was associated with a significantly shorter survival for both untreated and previously treated patients.136 In this study, elevated β2M was observed in patients with high tumor burden and extensive bone marrow infiltration. In addition to disease progression, both β2M and TKA have been associated with short duration of remission and inferior survival after treatment. Given the time frame of these studies, prospective validation with other biomarkers will be essential. Although the malignant cell of CLL morphologically resembles a mature lymphocyte, genetic, immunologic, and phenotypic studies suggest that this cell is better designated as either a pregerminal or post germinal B cell. Somatic mutations in the first and second complementarity-determining regions (CDR1 and CDR2) of the IGHV1 genes are thought to occur in the germinal centers. Examination of IGHV1 genes in patient cells suggest that there may be two subsets of CLL: leukemias whose cell of origin has successfully traversed the germinal center, resulting in the mutated IGHV1 phenotype and leukemias that are derived from naïve B cells with the nonmutated (germline) IGHV1 sequence. Approximately 60% of CLL patients have cells with mutated IGHV1 genes (<98% sequence identity to germline), whereas the remaining patients have cells exhibiting nonmutated IGHV1 (≥98% sequence identity with germline), typical of pre-germinal B cells. The prognostic significance of the absence of IGHV1 gene mutations is substantial, with all studies uniformly noting an inferior survival and high predisposition to requiring early treatment in this patient subset.25,26 This patient group has a higher frequency of clonal evolution, autoimmune complications, infections,137 and Richter transformation. A CLL Research Consortium study examined the impact of IGHV1 mutation in 307 untreated CLL patients enrolled on a prospective tissue collection study.138 Fifty-three percent of these patients exhibited nonmutated IGHV1 genes, and this population had a significantly shorter median time to initial therapy (3.5 years) than those with mutated IGHV1 genes (9.2 years, P < .001). Results from a second study of more than 1000 patients from the CLL Research Consortium validated this finding.139 Because of the difficulties in determining IGHV1 gene mutational status, researchers have sought surrogate markers for this parameter. Correlation between the absence of IGHV1 gene mutations and elevated expression of the cell surface molecule CD38 on CLL cells was noted in one such report.26 In another work, ZAP70 expression was shown to correlate with IGHV1 gene mutational status.138–142 Other surrogate markers for the mutational status of the IGHV1 gene have been reported, including elevated levels of lipoprotein lipase,40,41,143,144 and related genes have been noted in patients with IGHV1-nonmutated disease. Additionally, it has been reported that IGHV1-mutated CLL cells have long telomeres with low telomerase activity, whereas IGHV1-nonmutated cells have short telomeres with high telomerase activity.29–32,145 The extreme shortening of telomeres and elevated telomerase activity are associated with both genetic instability and disrupted apoptosis in other diseases, suggesting that a similar process occurs in IGHV1-nonmutated CLL cells. Multiple retrospective studies have shown that CD38 is an independent prognostic marker in CLL with high CD38 expression (7% to 30% positive tumor cells, depending on the series) being associated with both a shorter time from diagnosis to treatment and inferior survival.28,146–149 Unlike IGHV1 mutational status or ZAP70 expression, which remains relatively stable over the course of the disease in a given patient, CD38 expression status can change based on the compartment of disease localization and also over time with disease progression. Although CD38 expression was initially thought to be a good surrogate for IGHV1 mutational status,26 subsequent studies have shown a lower concordance and also that expression of this antigen changes over time.36,150 ZAP70 expression was identified as another surrogate marker for IGHV1 gene mutational status by a cDNA microarray analysis of untreated CLL patients.20 Multiple studies have shown that ZAP70 expression predicts for short time to treatment and also inferior survival.138–142,151 In one series reported by the CLL Research Consortium, the median time from initial diagnosis to treatment was 2.8 years, whereas for those without expression of ZAP70 it was 11 years.138 In several studies, multivariate analysis showed ZAP70 to be better at prediction than either IGHV1 mutational status or CD38139 and confirmed the time of diagnosis to treatment of patients with ZAP70 as being approximately 3 years. In addition to a poorer outcome, patients with ZAP70 also have a higher frequency of autoimmune complications arising from CLL.152 Despite clear data showing that ZAP70 is a prognostic factor, the reproducibility of this assay across laboratories has been problematic. Inconsistent measurement of ZAP70 may be the cause, because ZAP70 is a labile protein and laboratories have employed different methods and reagents. Given the challenges of measuring ZAP70 protein accurately, others have attempted to identify alternative markers or more stable readouts of ZAP70 expression. For example, methylation of select regions in the proximal 5′ region of the ZAP70 gene has been shown to correlate closely with expression of ZAP70 and influence treatment outcome.153,154 Our group recently completed a high-resolution, quantitative DNA methylation analysis of the entire ZAP70 gene regulatory region in 247 CLL patient samples from four separate clinical studies.155 Through this comprehensive analysis, we identified a small area in the 5′ regulatory region of ZAP70 with large variability in methylation. This sequence was found to be universally methylated in normal B cells. High correlation with mRNA and protein expression as well as activity in promoter reporter assays revealed that within this differentially methylated region, a single CpG dinucleotide and neighboring nucleotides are particularly important in ZAP70 transcriptional regulation. Furthermore, using clustering approaches, we identified a prognostic role for this site in four independent CLL patient data sets using time to treatment, PFS, and OS as clinical end points. Given the stability of genomic DNA methylation, resolution of quantification with this assay and high reproducibility of prognostic prediction across multiple clinical data sets, this test may facilitate rapid clinical assessment of ZAP70 status. Outside of such new tests, ZAP70 protein assessment by flow cytometry or other methods should still be used only in the setting of research. Conventional metaphase cytogenetics can identify chromosomal aberrations in only 20% to 50% of CLL cases because of the low in vitro mitotic activity of CLL cells.69 Abnormalities noted in descending frequency of occurrence include trisomy 12, deletions at 13q14, structural aberrations of 14q32, and deletions of 11q, 17p, and 6q. In addition, a complex karyotype (three or more abnormalities) occurs in approximately 15% of patients and predicts for rapid disease progression, Richter transformation, and inferior survival.156,157 A recent study showed that the use of CD40L or the combination of IL-2 and CpG stimulation revealed translocations in 33 of 96 patients (34%).73 These translocations were both balanced and unbalanced, occurring in 13q14, 11(q21q25), 14q32, or regions also seen in lymphomas such as 1(p32p36), 1(q21q25), 2(p11p13), 6(p11p12), 6(p21p25), and 18q21. These data define a new prognostic subgroup of patients with significantly shorter median time from diagnosis to requiring therapy (24 vs. 106 months) and OS (94 vs. 346 months) compared with those without translocations. The frequency of these translocations in untreated patients was less common, suggesting that these accumulate with disease progression. Given the limitation of standard or stimulated karyotype analysis, interphase cytogenetics of known abnormalities by FISH has become the current state-of-the-art technique for accurately distinguishing genetic subtypes of CLL. The largest study of interphase cytogenetics resulted in improved sensitivity to detect partial trisomies (12q12, 3q27, 8q24), deletions (13q14, 11q22-23, 6q21, 6q27, 17p13), and translocations (band 14q32) in more than 80% of all cases. In a large study of 325 patients by Dohner and colleagues, a hierarchical model consisting of five genetic subgroups was constructed on the basis of regression analysis of CLL patients with chromosomal aberrations.71 Patients with a 17p deletion had the shortest median survival time of 32 months and the shortest treatment-free interval (TFI) of 9 months, whereas patients with an 11q deletion followed closely with 79 months and 13 months, respectively. The favorable 13q14 deletion group had a long TFI of 92 months and a median survival of 133 months, whereas the group without detectable chromosomal anomalies and those with trisomy 12 fell into the intermediate group with median survival of 111 and 114 months, respectively, and TFI of 33 and 49 months, respectively. According to this pivotal study, CLL patients are prioritized in a hierarchical order (deletion 17p13 > deletion 11q22-q23 > trisomy 12 > no aberration > deletion 13q14).156 The hierarchical model of cytogenetic abnormalities in predicting disease progression by FISH has been further confirmed by other studies. Other much less frequently documented abnormalities including MYC translocations have been reported in less than 1% of CLL patients, generally with unfavorable prognostic features, short time to treatment, and short survival.158 The impact of high-risk interphase cytogenetics relative to disease progression, outside of its association with IGHV1-nonmutated CLL, in at least one study had less impact on outcome. Unlike other diseases, CLL has not yet had a common driving mutation that contributes significantly to the progression of the disease in a majority of patients. Until recently, the most common and well-characterized mutated gene in CLL was TP53, which often occurred in concert with del(17p13.1). The frequency of TP53 mutations and/or deletions at diagnosis of CLL is relatively infrequent (3% to 5%) but increases in patients with refractory disease when it may exceed 25%. Although approximately 75% of TP53 mutations occur together with del(17p13.1), a component of patients have isolated TP53 mutations without evidence of deletion in the other allele. Some studies have shown similar negative impact of these isolated point mutations in TP53 with respect to predicting time to treatment and also response to DNA-damaging agents such as alkylating agents and fludarabine.159–163 Many clinical studies are now including TP53 mutation screening as part of the standard risk stratification for early treatment intervention and also treatment stratification. Routine performance of TP53 mutations as part of all initial risk assessments at diagnosis and also at the time of treatment is not included outside of a clinical trial. Similarly, other recently identified recurring mutations such as NOTCH194,98,99 and also SF3B1 mutations have also shown frequency in high-risk genomic CLL of 10% or greater and may have prognostic significance.21,164 Further studies to prospectively validate these markers will be required before routine application of this assays can be recommended for clinical use in risk stratification. Until very recently there have been no published series that have integrated clinical, laboratory, and genomic features into a predictive model to identify patient risk for progressing onto early treatment as opposed to those who may have an extended period of observation. Wierda and colleagues from M.D. Anderson Cancer Center reported a prospective series of 930 patients who were newly diagnosed with the diagnosis of CLL who had examination, laboratory, and genomic assessment.125 With prospective follow-up, they identified each of the following criteria in a multivariate analysis to be significantly associated with progression: (1) three involved lymph node sites; (2) increased size of cervical lymph nodes; (3) the presence of del(17p13.1) or del(11q22.3); (4) increased serum lactate dehydrogenase level; and (5) nonmutated IGHV1 mutational status. A point algorithm to predict disease outcome using these features was established but requires validation from a second data set before widespread use can be recommended. Our understanding of the biology of CLL has improved dramatically, and many relevant biomarkers are now becoming useful for predicting when CLL will clinically progress. However, no study to date has demonstrated that earlier treatment will alter the natural history of the patient in even the higher-risk groups with high progression rates. Therefore, at the present time the use of staging and predictive biomarkers should be used only to provide patients with information relative to the expected course of their disease. Outside of a clinical trial, these results should never be used to initiate therapy in patients with asymptomatic disease and no indication for treatment. Before performing predictive tests, we generally discuss with the patient how we will use these tests and the potential consequences that can be derived from them. Specifically, for a subset of patients having low-risk disease at diagnosis with IGHV1-mutated disease and favorable karyotype, often significant relief occurs that allows resumption of regular life opportunities and more comfortable living with the diagnosis of CLL. However, in the patients who are identified to be at high risk for disease progression, significant anxiety can result after they are told they are at high risk for progression with ultimately expected short survival and yet no treatment or intervention will be pursued. With appropriate counseling, in our experience the majority of patients decide to have this testing whereas a small subset defers it. Currently such testing is recommended as optional and helpful by the NCCN130 and IWCLL 2008 guidelines.17 Box 102-1 provides an example of the initial evaluation provided by our group when seeing a newly diagnosed patient. It also includes a detailed discussion of complications that can arise as a consequence of CLL. Because the lymphocyte-doubling time is a prognostic feature in the progression of CLL,165,166 our approach is to follow patients every 3 months during the first year; if little change in clinical or laboratory parameters occurs at this point, we extend this time period to every 6 months in the absence of new complaints. Over the course of the disease, approximately 20% of patients with CLL will experience autoimmune hematologic complications that include, in descending order of occurrence, autoimmune hemolytic anemia (AIHA), idiopathic thrombocytopenia purpura (ITP), and pure red cell aplasia. In general, the majority of patients with CLL will develop these autoimmune complications when their disease is active, although a small proportion (10% to 15%) of patients have AIHA at diagnosis.167 AIHA has variable symptoms most often referable to profound anemia, including fatigue, weakness, lethargy, dyspnea on exertion, and dizziness. Rare presentations can include new clinically visible jaundice, headaches, or chest pain that identifies anemia on further evaluation. Although the insidious onset of AIHA can occur over 2 to 3 months, acute symptoms may occur that require rapid intervention. The typical laboratory findings include indirect hyperbilirubinemia, elevated lactate dehydrogenase (LDH) levels, low haptoglobin, and hemoglobinuria. The direct antiglobulin Coombs test (DAT) is positive in up to 75% of patients with CLL with AIHA. However, patients can have a positive DAT and not ultimately experience clinically significant hemolysis. Most of the red cell-directed antibodies produced are warm reactive (IgG antibodies), but patients can occasionally present with cold agglutination syndrome (IgM antibodies). The antibodies are mostly polyclonal and derived from residual normal B cells as compared with the malignant B-cell clone. The exception to this is IgM-derived antibodies, which are often monoclonal and derived from the malignant B-cell clone. Treatment of AIHA is derived from small case series and small nonrandomized studies.168,169 Glucocorticoids are considered the first line of therapy of AIHA. Prednisone 1 mg/kg is given for 14 to 28 days, followed by a slow taper over 2 to 3 months. Prophylaxis for opportunistic infections (e.g., from Pneumocystis jiroveci and varicella zoster virus) should be given to patients on extended prednisone therapy. The overall response rates to prednisone are as high as 90%, but unfortunately approximately 60% of patients experience relapse when corticosteroid therapy is tapered or discontinued. A poor response or inability to taper prednisone therapy within a reasonable time period (2 to 3 months) is a reason to initiate second-line therapy. Rituximab 375 mg/m2 weekly for 4 weeks is often effective for corticosteroid-resistant AIHA and also if corticosteroid withdrawal is not possible.172–172 Because of the long-term morbidity of prolonged corticosteroid administration in CLL and data demonstrating benefit to concurrent corticosteroids and rituximab for rapid withdrawal of corticosteroids in ITP,173 some physicians administer these agents concurrently at the diagnosis of AIHA unless a relative contraindication (i.e., hepatitis B) exists. Rituximab is also effective if a poor response to corticosteroids is noted.174,175 Intravenous immunoglobulin (IVIG), the next line of treatment, induces responses in 40% to 60% of patients with AIHA.176 Cyclosporine has been used for refractory AIHA of CLL at a dose of 5 mg/kg/day given in divided doses twice daily. Other treatment modalities include splenectomy177,178 or splenic irradiation,179 or chemoimmunotherapy180 if the disease is active. Treatment of the underlying CLL is generally required for long-term control of autoimmune cytopenias. However, our practice is to first control the autoimmune process with corticosteroids or other therapies before considering treatment of the underlying CLL. Supportive therapy with periodic red cell transfusions is also important in the management of AIHA. These cells should be irradiated and leukopore filtered to prevent development of transfusion-associated graft-versus-host disease. One poorly characterized type of AIHA relates to that observed during fludarabine therapy. Generally this occurs in the setting of no evident hemolysis before starting therapy with a fludarabine-based regimen. Time of onset is usually during the first three cycles with classic laboratory findings. With this particular type of drug-related AIHA, cessation of fludarabine is warranted and transitioning to an alternative regimen is best advised. Fludarabine-associated hemolytic anemia does recur with rechallenge, and fatalities in this setting have been described.181 Rechallenge with pentostatin has been reported in one case to trigger recurrent AIHA.182
Chronic Lymphocytic Leukemia
Introduction
Epidemiology
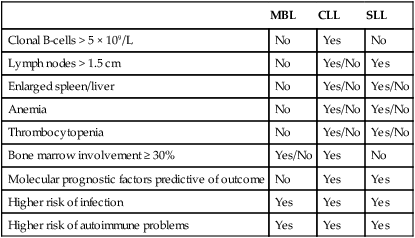
MBL
CLL
SLL
Clonal B-cells > 5 × 109/L
No
Yes
No
Lymph nodes > 1.5 cm
No
Yes/No
Yes
Enlarged spleen/liver
No
Yes/No
Yes/No
Anemia
No
Yes/No
Yes/No
Thrombocytopenia
No
Yes/No
Yes/No
Bone marrow involvement ≥ 30%
Yes/No
Yes
No
Molecular prognostic factors predictive of outcome
No
Yes
Yes
Higher risk of infection
Yes
Yes
Yes
Higher risk of autoimmune problems
Yes
Yes
Yes
Biology and Genetics
Is CLL a Stem Cell Disease?
Is IGHV1 Mutational Status the Differentiating Feature of Low- Versus High-Risk CLL?
Is ZAP70 Expression a Surrogate for IGHV1 Mutational Status or Driver Gene in the Pathogenesis of CLL?
B-Cell Receptor Signaling in the Pathogenesis of CLL
Is CLL a Disease of Defective Apoptosis?
Genetic Abnormalities
Recurring Mutations in CLL
Immune Suppression in Development and Progression of CLL
Contribution of Microenvironment in CLL Pathogenesis
Diagnosis
Staging and Prognostic Factors
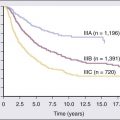
Stage
Lymphocytosis
Enlarged Nodes
Enlarged Spleen
Hgb < 11 g/dL
Platelets < 100 × 1012/L
Median Survival*
0
Yes
No
No
No
No
>150 months
1
Yes
Yes
No
No
No
101 months
2
Yes
Yes/No
Yes
No
No
71 months
3
Yes
Yes/No
Yes/No
Yes
No
19 months
4
Yes
Yes/No
Yes/No
Yes/No
Yes
19 months

Stage
Lymphocytosis
Three or More Nodal Areas Involved
Hgb < 10 g/dL or Platelets < 100 × 1012/L
Median Survival*
A
Yes
No
No
Similar to normal age-matched control
B
Yes
Yes
No
7 years
C
Yes
Yes/No
Yes
2 years

Imaging Studies and Predicting CLL Outcome
Thymidine Kinase Activity and β2-Microglobulin
IGHV1 Mutational Status
CD38 Expression
ZAP70
Chromosomal Aberrations
Select Gene Mutations
Integration of Clinical and Molecular Markers
How to Use Staging and Biomarkers
Complications
Autoimmune Complications
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Oncohema Key
Fastest Oncology & Hematology Insight Engine