Fig. 19.1
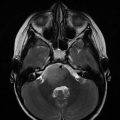
(a) H&E stained histological sample of a WHO Grade I Choroid Plexus Papilloma. There is a single layer of epithelial cells overlying a fibrovascular core, resembling normal choroid plexus, although demonstrating more cellular crowding. Mitotic activity is low and there is no evidence of necrosis. (b) H&E stained histological sample of a WHO Grade III Choroid Plexus Carcinoma. There is a loss of the papillary organization of the tissue as well as nuclear pleomorphism and atypia. While invasion of adjacent cerebral tissue is common, it is not identified on this sample. (Pathology images courtesy of Dr. DeMasters)
19.2 Predisposing Factors
CPTs, in particular CPC, frequently have mutations in TP53. CPCs are seen in patients with Li-Fraumeni syndrome, who have germline TP53 mutations and are one of the 2009 Chompret criteria (Tinat et al. 2009). A recent multi-institutional genomic analysis of 100 CPTs shows that CPCs differ from CPPs in copy number, gene expression, and DNA methylation (Merino et al. 2015). TP53 mutations were seen in a high proportion of CPC and the number of mutated TP53 was correlated with outcome. There may also be an association between simian virus 40 (SV40) and CPT. There is in vitro data (Carruba et al. 1983, 1984) and animal data (Brinster et al. 1984; Cho et al. 1989; Enjoji et al. 1996; Small et al. 1985) that shows SV40 can induce CPT.
19.3 Presenting Symptoms
In the pediatric population, the most common location for CPTs is the lateral ventricles, followed by the fourth ventricle. As a result, presenting symptoms often relate to elevated intracranial pressure (ICP), either as a result of obstructive hydrocephalus or CSF overproduction by tumor, which overwhelms the absorptive mechanisms. Signs and symptoms of elevated ICP include a full anterior fontanelle, irritability, lethargy headache (reported by verbal children), vomiting, and changes in vision. Visual changes are exceedingly difficult to detect in the pediatric population, but a downgaze preference or failure of a baby to track objects may indicate dysfunction. Ophthalmological examination may reveal papilledema. Additional physical exam findings in infants may include rapidly increasing head size or prominent scalp veins.
19.3.1 Radiographic Findings
Diagnostic imaging for children with CPTs usually includes computed tomography (CT) and magnetic resonance imaging (MRI), although infants with a rapidly increasing head circumference may also undergo an initial cranial ultrasound. Ultrasound is both noninvasive and inexpensive. It may reveal a dilated ventricular system and/or a hyperechoic mass. On CT, CPP is usually a well-defined mass within a dilated ventricular system. The tumor often has a frondular appearance and may or may not demonstrate calcification. On MRI, CPP again demonstrates a unique frondular appearance, consistent with its derivation from the choroid plexus. On both modalities, CPP avidly enhances following contrast administration. While adjacent edema may be identified with FLAIR or T2 sequences, significant edema raises concerns for local infiltration, which is most consistent with CPC. While CPP is very discrete (Fig. 19.2), CPC usually has less well-defined borders and is more heterogeneous in appearance, including cystic changes, necrosis, or hemorrhage (Fig. 19.3).
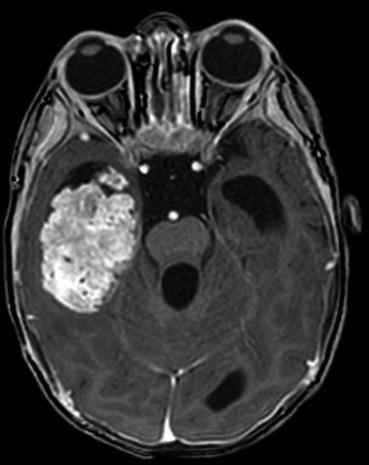
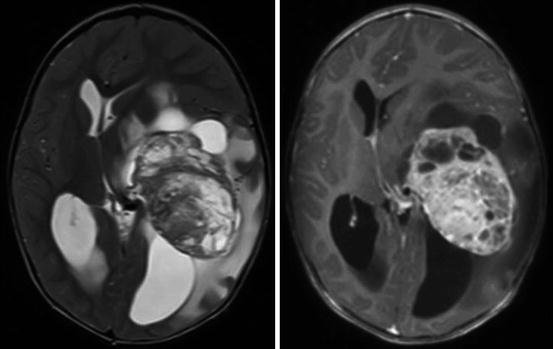

Fig. 19.2
Axial T1-weighted post-contrast MRI in a child with a CPP. The frondular tumor is located in the right temporal horn of the lateral ventricle, showing avid enhancement and no evidence of edema or infiltration involving the adjacent parenchyma

Fig. 19.3
Axial T2-weighted (left) and T1-weighted post-contrast (right) MRI in a child with a CPC. The tumor is located in the left lateral ventricle, showing less homogenous enhancement and multiple cysts. There is substantial edema in the adjacent temporal parenchyma
There is limited data regarding the use of other noninvasive imaging modalities in CPT. With MR spectroscopy, the choline peak may be more elevated in CPC compared to CPP (Horska et al. 2001) and myoinositol may be elevated in CPP compared to other choroid plexus tumors (Krieger et al. 2005). There have also been descriptions of using SPECT (Shibata et al. 2008; Wolff et al. 2001) and PET (Sunada et al. 2002). MR angiography is often a useful adjunct in revealing the vascular supply of the lesion, which can guide decision-making regarding surgery or the need for angiography with tumor embolization.
19.4 Workup
These suggested pretreatment evaluations are based on the CPT-SIOP-2009 protocol. Evaluation of these children begins with a history, physical examination, and basic bloodwork. Imaging should include MRI of the brain and complete spine. In the absence of elevated intracranial pressure (ICP), a lumbar puncture should also be performed to assess for metastatic disease. In the presence of elevated ICP, this may be completed postoperatively. Other metastatic evaluations are not typically needed. Other recommended evaluation includes EKG and ECHO for children who will receive doxorubicin, and audiology testing for children who will receive radiation to the cochlea or cisplatin.
19.5 Acute Management/Surgical Treatment
Many children with CPTs present with symptomatic ICP elevation. As a result, initial management often focuses on this issue, including the administration of mannitol or the urgent/emergent placement of an external ventricular drain or ventricular shunt. Typically, third ventriculostomy is less effective due to overproduction of CSF. During the preoperative period, patients are commonly maintained on dexamethasone.
The goal of primary management of both CPP and CPC is maximal, safe surgical resection. As a highly vascular lesion that commonly presents in very young small children, the risk of life-threatening intraoperative blood loss must always be considered. Based on the radiographic appearance of the lesion, the clinical team determines whether preoperative angiography with embolization is indicated. While this may be challenging in the very young, embolization can substantially reduce the arterial supply of the tumor that is particularly relevant in suspected CPC.
The tumor is approached using a stereotactically guided craniotomy with a transventricular trajectory that provides early access to the tumor’s arterial supply, which is then cauterized and cut. The need for stereotactic guidance and stability of the operative field during microsurgical resection may require specialized head holders to accommodate the thin skull of the very young. The risk of skull fracture and epidural hematoma must always be carefully evaluated by the neurosurgeon during operative positioning and following surgery, as the usual self-tamponade of a small epidural hematoma is lost following the removal of CSF and tumor. Close communication between the operating team and anesthesiologist is critical in order to prevent rapid and catastrophic vascular collapse as a result of intraoperative blood loss and fluid shifts. The operative approach is tailored to the location of the lesion and the vascular supply, which invariably includes choroidal vessels. Unlike the case with many intracranial tumors, internal tumor debulking is not the recommended approach for CPTs, as this could result in dramatic tumoral hemorrhage. Instead, the surgeon carefully disconnects the arterial supply with the goal of removing the tumor en bloc. Due to the vascularity of these lesions, safe tumor removal may require staged resection, especially in the case of CPC, which is more likely to be infiltrative, creating challenges for en bloc resection.
Following tumor resection, an external ventricular drain is invariably left in place. This may be weaned and removed in some cases, but permanent CSF diversion through ventriculo-peritoneal shunting is often required. Positioning of the shunt should take into account the possibility that a young child will collateralize some neurological function to the non-injured contralateral hemisphere.
19.6 Postoperative Management
Outcomes for CPP after surgery alone show excellent results. In the Canadian Pediatric Brain Tumor Consortium, all 21 children with CPP were alive after surgery alone (Lafay-Cousin et al. 2011). Extent of surgery is also correlated with improved outcomes for CPC. A meta-analysis of 347 CPC patients found 5-year overall survival (OS) of 58.1% for completely resected CPC compared to 20.9% for those with residual disease (Wrede et al. 2007). A more recent meta-analysis of 322 patients from 101 studies found similar improvements in outcomes with extent of surgery (Sun et al. 2014).
Given the low incidence of CPTs, treatment recommendations for adjuvant therapy or observation after resection are somewhat variable. Here we summarize recommendations based on SIOP-CPT-2009. For CPP, regardless of extent of resection or completely resected localized aCPP, observation is recommended. For aCPP with metastatic disease, and all CPC, adjuvant chemotherapy is recommended. In the previously mentioned meta-analysis, for CPC chemotherapy was associated with improved 5-year OS, 46.4% for those receiving chemotherapy compared to 27.6% for no chemotherapy. High dose chemotherapy with autologous stem-cell rescue has been used in the Head Start regimens (Zaky et al. 2015). In that series of 12 children, 5-year OS and PFS was 62% and 38%, respectively. CPT-SIOP-2000, which enrolled 131 patients, compared carboplatin/etoposide/vincristine to cyclophosphamide/etoposide/vincristine and found no difference. CPT-SIOP-2009 is now studying additional agents including doxorubicin, actinomycin D, cisplatin, methotrexate, temozolomide, irinotecan, intrathecal etoposide, and intrathecal cytarabine.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree