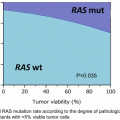
Fig. 12.1
Anatomic location of cholangiocarcinoma subtypes: CCA can be classified as being intrahepatic cholangiocarcinoma (ICC) or extrahepatic cholangiocarcinoma (ECC), based on the location of the tumor. ECC is divided into perihilar and distal cholangiocarcinoma. By definition, perihilar ECC are those that arise above the confluence of cystic and common hepatic duct, mid-bile duct are those that arise between the confluence of cystic duct and common bile duct and the upper border of the duodenum, and distal CCA are those located in the duodenal and intrapancreatic portion of bile duct up to the ampulla of Vater. Intrahepatic cholangiocarcinoma (ICC) is variably defined as arising from the second or more distal branches of the intrahepatic bile ducts or involving the intrahepatic ducts not extending into the hepatic hilum (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Although ICC and ECC are often histologically indistinguishable, they are distinct from one another in their presentation, prognostic factors, and growth characteristics [5, 6]. Patients with ICC typically present with abdominal pain and an intrahepatic mass, whereas patients with ECC present with painless jaundice [7]. CCA should be considered in any patient who presents with obstructive jaundice. This review will focus on the surgical treatments and management of both ECC and ICC.
Incidence
CCA is an uncommon tumor with an overall incidence about 1 in 100,000 people annually in the United States. The majority of these tumors occur at the hepatic duct bifurcation (60–80 %). ICC is the second most common primary hepatic malignancy representing 5–15 % of all tumors [5, 8]. There is a marked geographic variability in the incidence of cholangiocarcinoma, the highest occurring in the Far East. The incidence of ICC in the United States and the United Kingdom has risen recently, in contrast to ECC, which appears to be decreasing [9, 10]. The rise in ICC incidence may be related to better awareness of the disease and improved diagnosis with immunohistochemistry techniques. Another factor that may contribute to ICC rising incidence is the increased immigration from high prevalent regions of the world [9, 11].
Etiology
A number of risk factors have been linked to CCA, and they are parasitic infections (e.g., Clonorchis sinensis and Opisthorchis viverrini) which are endemic in Japan and Southeast Asia, hepatolithiasis, diabetes, smoking, cirrhosis, hepatitis C virus (HCV), prior biliary-enteric anastomosis, and congenital choledochal cyst [4, 12, 13]. Other predisposing conditions include primary sclerosing cholangitis (PSC), Caroli’s disease, and exposure to the contrast-medium thorotrast [4, 7, 14]. Chronic inflammation and/or injury to the bile duct epithelium are a common theme in all of these conditions (Table 12.1). The most common predisposing condition associated with CCA in the Western hemisphere is PSC, with a reported lifetime incidence of CCA between 6 and 10 % [7, 15].
Table 12.1
Comparison of preexisting medical conditions among patients with extrahepatic cholangiocarcinoma, intrahepatic cholangiocarcinoma, and controls
ECC (n = 549) | ICC (n = 535) | Controls (n = 102,782) | ||||||
|---|---|---|---|---|---|---|---|---|
Condition | N | % | P valuea | N | % | P valuea | N | % |
Bilary tract conditions/operations | ||||||||
Choledochal cysts | 27 | 4.9 | <0.001 | 21 | 3.9 | <0.001 | 108 | 0.1 |
Cholangitis | 50 | 9.1 | <0.001 | 67 | 12.5 | <0.001 | 201 | 0.2 |
Biliary cirrhosis | <5 | <0.9 | 0.003 | 5 | 0.9 | <0.001 | 53 | 0.1 |
Cholelithiasis | 202 | 36.8 | <0.001 | 172 | 32.1 | <0.001 | 4,273 | 4.2 |
Choledocholithiasis | 87 | 15.8 | <0.001 | 59 | 11 | <0.001 | 543 | 0.5 |
Cholecystitis | 42 | 7.7 | <0.001 | 29 | 5.4 | <0.001 | 973 | 0.9 |
Cholecystectomy | 87 | 15.8 | <0.001 | 41 | 7.7 | <0.001 | 1,649 | 1.6 |
Chronic liver diseases | ||||||||
Alcoholic liver disease | 8 | 1.5 | <0.001 | 5 | 0.9 | 0.008 | 310 | 0.3 |
Nonspecific cirrhosis | 10 | 2 | <0.001 | 17 | 3.2 | <0.001 | 359 | 0.3 |
8 | ||||||||
Hemochromatosis | <5 | <0.9 | 0.25 | <5 | <0.9 | 0.05 | 282 | 0.3 |
Chronic nonalcoholic liver disease | <5 | <0.9 | 0.08 | 5 | 0.9 | 0.03 | 353 | 0.3 |
HCV infection | <5 | <0.9 | 0.36 | <5 | <0.9 | 0.03 | 142 | 0.1 |
Endocrine disorders | ||||||||
Diabetes mellitus type II | 165 | 30.1 | <0.001 | 177 | 33.1 | <0.001 | 22,764 | 22.1 |
Thyrotoxicosis | 30 | 5.5 | 0.04 | 27 | 5 | 0.12 | 3,864 | 3.8 |
Digestive disorders | ||||||||
IBD | 10 | 1.8 | 0.03 | 18 | 3.4 | <0.001 | 936 | 0.9 |
Crohn’s disease | 6 | 1.1 | 0.02 | 5 | 0.9 | 0.06 | 419 | 0.4 |
Ulcerative colitis | 5 | 0.9 | 0.11 | 13 | 2.4 | <0.001 | 595 | 0.6 |
Duodenal ulcer | 20 | 3.6 | 0.001 | 34 | 6.4 | <0.001 | 1,836 | 1.8 |
Chronic pancreatitis | 13 | 2.4 | <0.001 | 8 | 1.5 | <0.001 | 272 | 0.3 |
Miscellaneous conditions | ||||||||
Smoking | 12 | 2.2 | 0.03 | 12 | 2.2 | 0.02 | 1,212 | 1.2 |
Obesity | 16 | 2.9 | 0.79 | 23 | 4.3 | 0.12 | 3,201 | 3.1 |
Presentation, Diagnosis, and Assessment of Resectability
Painless jaundice is the most common presentation of ECC (i.e., perihilar or distal tumors). Patients with ICC typically present with abdominal pain, fever/chills, or an incidental liver mass found on imaging studies during a work-up for something else [5]. There are no specific blood tests or tumor markers that are diagnostic for CCA. However, a CA 19-9 level greater than 100 U/ml has been reported to predict the likelihood of malignancy in patients with PSC (sensitivity: 75–89 %; specificity: 80–86 %) and risk of recurrence after surgical resection [16]. When such a rise in CA 19-9 occurs in a patient without a history of PSC, the sensitivity decreases to 53–68 % but specificity increases up to 87 % [17, 18].
Carcinoembryonic antigen (CEA) is commonly monitored along with CA 19-9 when the diagnosis of cholangiocarcinoma is being entertained. Elevation of either CA 19-9 or CEA in isolation is not helpful, but in the proper clinical setting, it may increase the accuracy of diagnosing CCA. A Ramage score, which combines CA 19-9 and CEA, when elevated in a patient with PSC has a sensitivity and specificity of 71 % and 91 %, respectively [19].
Typically at the time of presentation, patients with ECC tend to have a total serum bilirubin above 10 mg/dL. An elevated alkaline phosphatase level higher than 5 times normal usually accompanies the elevated bilirubin levels, although these values are not specific enough for making the diagnosis. Patients can present with symptoms associated with cholestasis, such as pruritus, cholangitis, pale stool, and weight loss [20].
Radiologic evaluation includes ultrasound (U/S), computed tomography (CT), magnetic resonance imaging (MRI) and/or multi-detector-row computed tomography (MDCT), and magnetic resonance cholangiopancreatography (MRCP). Data on positron-emission tomography (PET) is limited. Ultrasound (U/S) has limited utility in diagnosis CCA, although it is often the first test performed. U/S is helpful in patients with obstructive jaundice so as to exclude the diagnosis of cholelithiasis and choledocholithiasis, since these conditions are more common than ECC.
In many centers, triphasic CT scan is the diagnostic test of choice to visualize the cancer and assess its relationship with adjacent vascular structures. CT is also useful to stage the patient since it could detect distant metastases. ICC typically shows hyperattenuation on delayed intravenous contrast images because of interstitial uptake of contrast medium in the tumor [21, 22] (Figs. 12.2 and 12.3). The percentage of tumor volume showing delayed uptake has been shown to correlate with increased fibrous stroma, perineural invasion, and worse prognosis [23]. On CT scans, both ICC and metastatic colorectal cancers have central hypointensity, but ICC typically has peritumoral biliary dilatation.
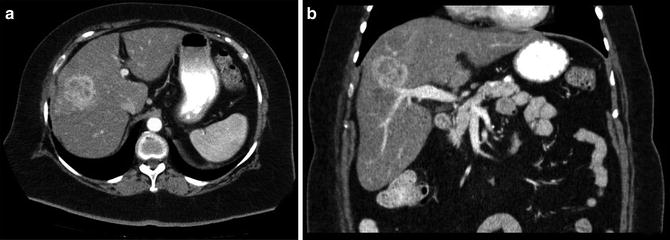
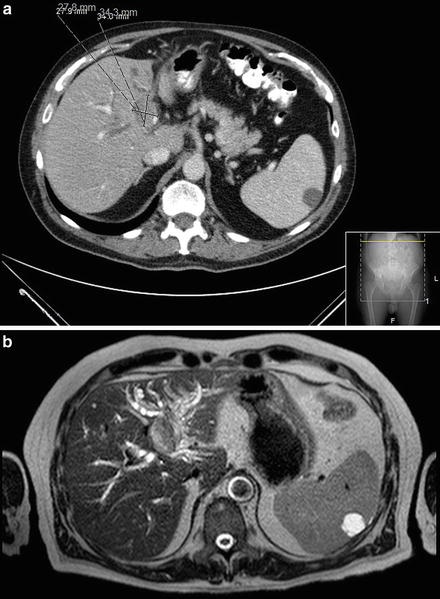

Fig. 12.2
A 72-year-old woman with an intrahepatic cholangiocarcinoma of the right lobe of the liver (a) axial and (b) coronal CT images. She underwent a successful right hepatectomy (Courtesy of Gazi B. Zibari, MD, FACS)

Fig. 12.3
A 73-year-old man with intrahepatic cholangiocarcinoma of the left lobe of the liver. (a) CT scan, (b) MRI. He underwent a successful left hepatectomy (Courtesy of Gazi B. Zibari, MD, FACS)
A hilar cholangiocarcinoma will have a picture of dilated intrahepatic biliary tree, with normal or collapsed gallbladder and extrahepatic biliary tree. Distal tumors present with dilation of the gallbladder and both the extra- and intrahepatic biliary tree. In other centers, MRI is the diagnostic imaging of choice. MRI is very accurate in delineating the longitudinal and lateral spread of extrahepatic cholangiocarcinoma and determining resectability. MRCP has also been utilized in evaluating patients with CCA (Fig. 12.4). It is accurate in defining the biliary tree, determining the site of obstruction, and is comparable to endoscopic retrograde cholangiopancreatography (ERCP) [24]. Both MRI and MRCP are the diagnostic of choice for perihilar cholangiocarcinoma.
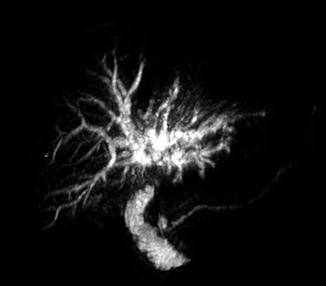

Fig. 12.4
MRCP of a patient with Klatskin’s tumor (Courtesy of Gazi B. Zibari, MD, FACS)
As already mentioned, ICC generally presents as a solid liver mass. As such, a preoperative tissue diagnosis is often not necessary. The mass represents either a metastatic lesion (which is a more common situation) or a primary liver tumor. Diagnostic work-up for such a mass generally entails a search for a primary which includes a colonoscopy and upper endoscopy to rule out a gastrointestinal source, review of a chest X-ray to rule-out primary lung, and for women, a mammogram and examination of the breast to exclude breast cancer. In addition, a careful review of CT scans, preferably with a radiologist, is essential not only to characterize the liver lesion but also to evaluate the pancreas and other solid organs as possible primary tumors that give rise to the liver lesion.
It is not uncommon for the surgeon to be asked to evaluate a patient who already had a liver biopsy of a solid mass and the pathology reveals an adenocarcinoma. In such a case, the possibility of an ICC should be entertained if there is no obvious primary tumor. Because it is difficult to differentiate ICC adenocarcinoma from metastatic adenocarcinoma, a panel of immunohistochemistry stainings can be helpful. Although there is no specific stain for ICC, a particular pattern of stains can assist at making the diagnosis (positive, CK7+, CK20- with biliary epithelial dysplasia, AE1/AE3; negative, TTF1, CDX2, DPC4) [25]. Regardless, the majority of patients with liver masses should be considered for surgical resection after a proper work-up has been performed. ICC is generally diagnosed after the surgery.
For patients with extrahepatic cholangiocarcinoma, a preoperative tissue diagnosis is also not necessary for surgical intervention. Patients who present with jaundice and dilated biliary tree but have no evidence of biliary stones or intrinsic hepatic disease should be considered to have a malignancy unless proven otherwise. Most hilar strictures are cholangiocarcinoma although 10–15 % of patients may have alternative diagnosis such as gallbladder cancer, Mirizzi syndrome, and idiopathic benign focal stenosis [26]. Gallbladder cancer generally presents with a thickened, irregular, and distended gallbladder, whereas hilar cholangiocarcinoma generally presents with a shrunken gallbladder. Mirizzi syndrome is a benign condition whereby a large gallstone that is impacted in the cystic duct or neck of the gallbladder causes extrinsic compression against the common hepatic duct [27].
Benign biliary strictures at the hepatic duct confluence are rare and can often mimic malignancy. Unfortunately, there is no diagnostic test that accurately distinguishes biliary strictures from malignancy. Thus, a negative biopsy does not necessarily rule out the possibility of a cancer, and therefore, surgeons should be cognizant of this and carefully weigh the risk and benefits of observation versus resection. As a general rule, in the absence of a clear contraindication, all hilar strictures/lesions should be considered for surgical exploration (Fig. 12.5).
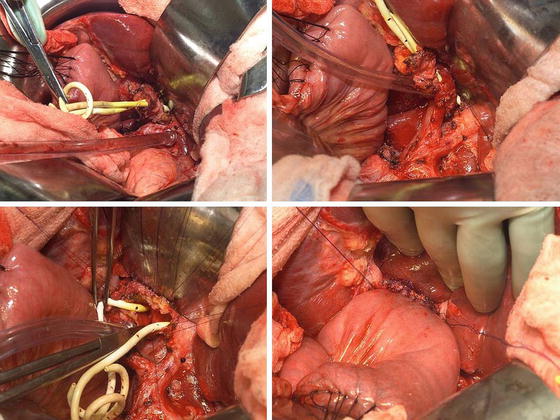

Fig. 12.5
A patient with biliary stricture at the hepatic duct bifurcation. Preoperative work-up was nondiagnostic. She underwent resection and a Roux-en-Y hepaticojejunostomy. The final pathology was a benign lesion (Courtesy of Gazi B. Zibari, MD, FACS)
For patients with obstructive jaundice without evidence of a hilar stricture or a mass in the head of the pancreas, the possibility of distal cholangiocarcinoma should be considered. Endoscopic ultrasound with biopsy can be helpful, although a negative biopsy does not exclude carcinoma and/or alter management in those who have no contraindications for surgery.
Staging and Classification
Staging of Intrahepatic Cholangiocarcinoma
Several staging and classification systems are currently being used for ICC, but the American Joint Committee on Cancer (AJCC/International) Union against Cancer (UICC) TNM classification system is the one that is widely used in the United States (Table 12.2). Historically, the staging systems for ICC and hepatocellular carcinoma were identical. However, the latest 7th edition of (AJCC/UICC) separated ICC into a separate entity based on data involving nearly 600 patients from the Surveillance, Epidemiology, and End Results (SEER) database as well as other international multicenter data [29–31]. Note that tumor size has no prognostic impact in ICC.
Table 12.2
American Joint Committee on Cancer (AJCC) TNM staging for intrahepatic bile ducts (7th edition)
Primary tumor (T) | |
|---|---|
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ (intraductal tumor) |
T1 | Solitary tumor without vascular invasion |
T2a | Solitary tumor with vascular invasion |
T2b | Multiple tumors, with or without vascular invasion |
T3 | Tumor perforating the visceral peritoneum or involving the local extrahepatic structures by direct invasion |
T4 | Tumor with periductal invasion |
Regional lymph nodes (N) | |
|---|---|
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis present |
Distant metastasis (M) | |
|---|---|
M0 | No distant metastasis |
M1 | Distant metastasis present |
Anatomic stage/prognostic groups | |||
|---|---|---|---|
Group | T | N | M |
Stage 0 | Tis | N0 | M0 |
Stage 1 | T1 | N0 | M0 |
Stage II | T2 | N0 | M0 |
Stage III | T3 | N0 | M0 |
Stage IVA | T4 | N0 | M0 |
Stage IVB | Any T | N1 | M0 |
Any T | Any N | M1 | |
The Liver Cancer Study Group of Japan (LCSGJ) developed a classification system from more than 240 resected cases of ICC that is specifically based on macroscopic appearance: mass forming (MF), periductal infiltrating (PI), and intraductal growth (IG) [32] (Fig. 12.6). MF type forms a definite mass within the liver parenchyma. The PI type extends mainly longitudinally along the bile duct and often resulting in dilatation of the peripheral bile duct. The IG type proliferates toward the lumen of the bile duct papillarity or like a tumor thrombus [32]. Tumors can have more than one macroscopic appearance and in such cases, they are described with the predominant type mentioned first followed by the subordinate type separated by a “+” (i.e., mass forming + periductal infiltrating).
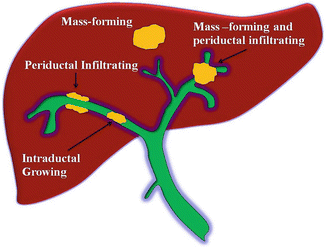

Fig. 12.6
The Liver Cancer Study Group of Japan (LCSGJ) developed a classification system of ICC: mass forming (MF), periductal infiltrating (PI), and intraductal growth (IG). MF type forms a definite mass within the liver parenchyma. The PI type extends mainly longitudinally along the bile duct and often resulting in dilatation of the peripheral bile duct. The IG type proliferates toward the lumen of the bile duct papillarity or like a tumor thrombus. Tumors can have more than one macroscopic appearance, and in such cases, they are described with the predominant type mentioned first followed by the subordinate type separated by a “+” (i.e., mass forming + periductal infiltrating) (Courtesy of Quyen D. Chu, MD, MBA, FACS)
MF is the most common subtype, representing approximately 60–80 % of ICC. PI and IG make up 15–35 % and 8–29 % of ICC, respectively. The macroscopic appearance of the disease reflects tumor cells with different biologic behavior. The biologic difference of these types is underscored by the differences in the 1-year overall survival (OS) rate of patients with ICC. MF has the best prognosis while MF + PI has the worst; the 1-year OS for MF, PI, and MF + PI are 80 %, 69, and 39 %, respectively (P = 0.0072) [33].
Staging of Extrahepatic Cholangiocarcinoma
Perihilar cholangiocarcinomas are also staged according to the AJCC/UICC TNM system (Table 12.3), although they are more commonly classified according to their anatomic location and extent of ductal infiltration based on the Bismuth-Corlette classification (Fig. 12.7). Such a classification is useful because it approaches perihilar cholangiocarcinomas from a surgical perspective; the location and extent of disease determines how one surgically approach and resect the tumor. Type I tumors are limited to or confined to the common hepatic duct, type II involve the confluence of the right and left hepatic ducts, and types IIIa and IIIb are tumors of common hepatic duct that extend to either the right (IIIa) or left hepatic duct (IIIb). Type IV tumors involve both the secondary bile ducts on both right and left hepatic duct [35, 36]. For types I–IIIa Bismuth-Corlette hilar cholangiocarcinoma, an extended right hepatectomy is the procedure of choice, whereas a left or extended left hepatectomy is used for type IIIb hilar cholangiocarcinoma [37].
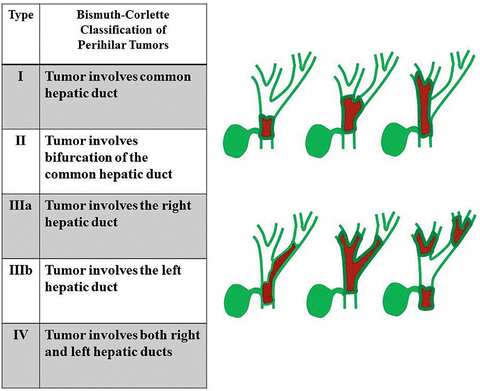
Table 12.3
American Joint Committee on Cancer (AJCC) TNM staging for perihilar bile ducts (7th edition)
Primary tumor (T) | |
|---|---|
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ |
T1 | Tumor confined to the bile duct, with extension up to the muscle layer or fibrous tissue |
T2a | Tumor invades beyond the wall of the bile duct to surrounding adipose tissue |
T2b | Tumor invades adjacent hepatic parenchyma |
T3 | Tumor invades unilateral branches of the portal vein or hepatic artery |
T4 | Tumor invades main portal vein or its branches bilaterally; or the common hepatic artery; or the second-order biliary radicals bilaterally; or unilateral second-order biliary radicals with contralateral portal vein or hepatic artery involvement |
Regional lymph nodes (N) | |
|---|---|
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis (including nodes along the cystic duct, common bile duct, hepatic artery, and portal vein) |
N2 | Metastasis to periaortic, pericaval, superior mesenteric artery, and/or celiac artery lymph nodes |
Distant metastasis (M) | |
|---|---|
M0 | No distant metastasis |
M1 | Distant metastasis present |
Anatomic stage/prognostic groups | |||
|---|---|---|---|
Group | T | N | M |
Stage 0 | Tis | N0 | M0 |
Stage 1 | T1 | N0 | M0 |
Stage II | T2a-b | N0 | M0 |
Stage IIIA | T3 | N0 | M0 |
Stage IIIB | T1-3 | N1 | M0 |
Stage IVA | T4 | N0-1 | M0 |
Stage IVB | Any T | N2 | M0 |
Any T | Any N | M1 | |

Fig. 12.7
Bismuth-Corlette classification for hilar cholangiocarcinoma
Distal cholangiocarcinoma is staged using the AJCC/UICC TNM classification system (Table 12.4).
Table 12.4
American Joint Committee on Cancer (AJCC) TNM staging for distal bile duct (7th edition)
Primary tumor (T) | |
|---|---|
TX | Primary tumor cannot be assessed |
T0 | No evidence of Primary tumor |
Tis | Carcinoma in situ |
T1 | Tumor confined to the bile duct histologically |
T2 | Tumor invades beyond the wall of the bile duct |
T3 | Tumor invades the gallbladder, pancreas, duodenum, or other adjacent organs without involvement of the celiac axis, or the superior mesenteric artery |
T4 | Tumor involves the celiac axis, or the superior mesenteric artery |
Regional lymph nodes (N) | |
|---|---|
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis |
Distant metastasis (M) | |
|---|---|
M0 | No distant metastasis |
M1 | Distant metastasis present |
Anatomic stage/prognostic groups | |||
|---|---|---|---|
Group | T | N | M |
Stage 0 | Tis | N0 | M0 |
Stage IA | T1 | N0 | M0 |
Stage IB | T2 | N0 | M0 |
Stage IIA | T3 | N0 | M0 |
Stage IIB | T1 | N1 | M0 |
T2 | N1 | M0 | |
T3 | N1 | M0 | |
Stage III | T4 | Any N | M0 |
Stage IV | Any T | Any N | M1 |
Treatment
Curative resection offers the best chance for long-term survival. Whereas palliation with surgical bypass was once the preferred surgical procedure even for resectable disease, aggressive surgical resection is now the standard. Despite a thorough preoperative radiologic evaluation to identify resectable disease, the rate of patients found to have unresectable disease at laparotomy remains 14–38 % [39–42]. Because of the relatively high rate of unresectability and the fact that survival of patients with incomplete resection is the same as those who were palliated conservatively, diagnostic laparoscopy has been used by several institutions as part of the staging process (Fig. 12.8). For patients who are known to have unresectable disease preoperatively, biliary endoprosthesis (i.e., endoscopic or percutaneous stenting) has supplanted the need for a surgical bypass.
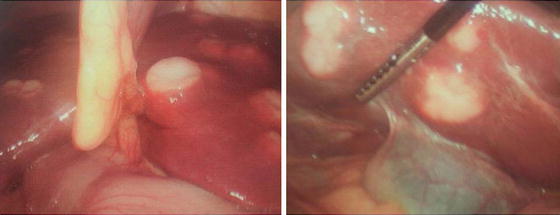

Fig. 12.8
A 65-year-old woman with multiple hepatic lesions that were suspicious for intrahepatic cholangiocarcinoma. Preoperative biopsy was nondiagnostic. She underwent a diagnostic laparoscopy and was found to have satellite lesions (Courtesy of John F. Gibbs, MD, FACS)
The role for preoperative biliary decompression with a stent for patients with resectable disease is controversial. For those who have asymptomatic biliary obstruction who will undergo surgery within 1–2 weeks, a stent is not advisable. For symptomatic patients or those who will undergo chemotherapy before surgery, a stent is advisable.
Intrahepatic Cholangiocarcinoma (ICC)
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver tumor. Surgical management of intrahepatic cholangiocarcinoma includes consideration for portal vein embolization (PVE), lymphadenectomy, extended hepatic resection with or without vascular resection, and orthotopic liver transplantation.
Resection of ICC follows the basic principles of anatomic liver resection for malignant neoplasm. The understanding of hepatic anatomy began in 1654 with Glisson’s description of the liver capsule. This was followed by Cantlie’s description of the division of the liver into functional halves in 1897. Finally, Couinaud defined the segmental anatomy of the liver in 1957 [43] (Fig. 12.9). The anatomic division of the liver into right and left halves is based on Cantlie’s line, which is an imaginary line that extends from the gallbladder fossa to the left of the vena cava. Anatomically this is defined by the middle hepatic vein. Each half of the liver is then divided into four other segments based on the venous drainage, portal venous inflow, and arterial inflow. The caudate lobe (segment I) is separate from the left and right hepatic lobes. A thorough understanding of the hepatic segmental anatomy increases the safety of the liver resection and an anatomic resection has been shown to result in increased survival [44].
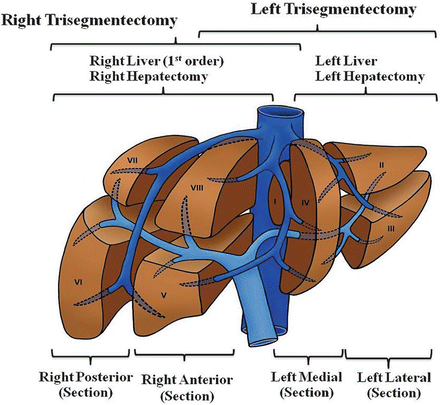

Fig. 12.9
Couinaud’s anatomic/segmental divisions of the liver and types of liver resection. The anatomic divisions of the liver according to the Brisbane 2000 terminology, including first-order divisions (hemilivers), second-order divisions (sections), and third-order divisions (segments) (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Table 12.5 shows one of the largest reported series in the literature examining outcome with hepatectomy for ICC. The 5-year overall survival is 23–40 % for resected patients [2–4, 39–41, 47, 49, 50, 52, 54–56]. The OS for an R0 resection is 36–63 % at 5 years and the postoperative mortality rate is 0–8 % [2, 3, 52]. In series with multivariate analysis, the most frequently cited significant negative prognostic factors are positive margin, satellite lesions, lymph node metastasis, lymphatic invasion, and vascular invasion. Of these, lymphatic and microvascular invasions are not helpful in selecting patients for resection because they cannot readily be determined preoperatively.
Table 12.5
Outcomes after resection for intrahepatic cholangiocarcinoma
Study | Year | N | Postop mortality (%) | Median survival (mos) | Median survival RO (mos) | 1–year OS (%) | 3-year OS (%) | 5-year OS (%) | 5-year OS RO (%) |
|---|---|---|---|---|---|---|---|---|---|
Jan [4] | 1996 | 41 | 0 | 12 | 22 | 54 | 37 | 26 | 44 |
Casavilla [45] | 1997 | 34 | 38 | 60 | 37 | 31 | 45 | ||
Chu [46] | 1999 | 48 | 16 | 60 | 30 | 22 | |||
Valverde [42] | 1999 | 30 | 3 | 28 | 86 | 22 | |||
Weimann [47] | 2000 | 95 | 5 | 18 | 64 | 31 | 21 | ||
Inoue [48] | 2000 | 52 | 18 | 63 | 36 | 36 | 55 | ||
Shimada [49] | 2001 | 49 | 4 | 26 | 66 | ||||
Okabayashi [50]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|