- 13.1 Introduction
- 13.2 Respiratory infections
- 13.2.1 Infection in the immunocompetent host
- 13.2.2 Infection in the immunocompromised host
- 13.2.1 Infection in the immunocompetent host
- 13.3 Granulomatous diseases
- 13.3.1 Formation of a granuloma
- 13.3.2 Tuberculosis
- 13.3.3 Sarcoidosis
- 13.3.1 Formation of a granuloma
- 13.4 Interstitial lung disease
- 13.4.1 Pulmonary eosinophilia
- 13.4.2 Extrinsic allergic alveolitis
- 13.4.3 Idiopathic interstitial pneumonias
- 13.4.1 Pulmonary eosinophilia
- 13.5 Connective tissue disease and the lung
- 13.6 Pulmonary vasculitis
- 13.6.1 Granulomatosis with polyangitis (previously Wegener’s)
- 13.7 Cardiac disease
- 13.7.1 Pericarditis
- 13.7.2 Myocarditis
- 13.7.3 Endocarditis
- 13.7.1 Pericarditis
- 13.8 Coronary artery disease
- 13.9 Diseases of the great vessels
 Visit the companion website at www.immunologyclinic.com to download cases with additional figures on these topics.
Visit the companion website at www.immunologyclinic.com to download cases with additional figures on these topics.
13.1 Introduction
Antigen can enter the respiratory tract either in the inspired air or via the circulation. Organisms that enter through the airways may be killed by local defence mechanisms, persist in the lung with damaging consequences (such as granuloma or fibrosis) or invade the systemic circulation to cause septicaemia. Since all the blood from the right side of the heart passes through the pulmonary bed, the respiratory tract is also exposed to circulating organisms, immune complexes and toxic substances from distant sites.
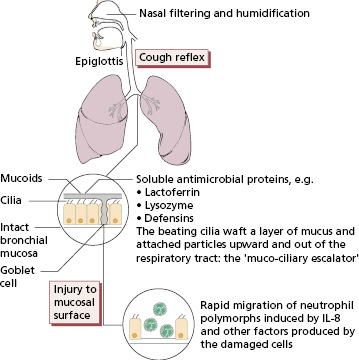
The respiratory tract can be crudely but usefully divided into two anatomical, functional and pathological compartments: the airways (from the nose to the terminal bronchiole) and the air spaces (or alveoli). The airways are protected from inhaled microorganisms and other potentially injurious particles by multiple mechanical factors, backed up by soluble antimicrobial proteins and rapid recruitment of neutrophils and other inflammatory cells (Fig. 13.1). Access to the alveolar compartment is therefore usually limited to very small inhaled particles (<5 μm diameter) (see Fig. 4.4) and organisms or antigen carried via the pulmonary circulation. Particles and organisms gaining access to the alveoli encounter further protective mechanisms such as the surfactant proteins (which have a complement-like function) and alveolar macrophages. Alveolar macrophages are responsible for ingesting, killing and degrading foreign material, both living and dead. Although their action in removing organisms from the lower respiratory tract is crucial, their reaction to inert materials can sometimes cause pulmonary damage. Viable cells from the alveoli can be recovered by bronchoalveolar lavage (BAL). Macrophages constitute 80–85% of cells in BAL fluid, while lymphocytes (mostly T cells) constitute about 10%. Cigarette smoking increases the cell yield by four- to five-fold and the macrophages are filled with tars and silicates. The proportion of neutrophils (10–20%) in BAL is also increased in smokers compared with non-smokers (2–5%), and most are activated, containing a high concentration of neutrophil elastase, which may play a role in the lung destruction seen in chronic bronchitis and emphysema.
Antigen-specific immune components are also found in the respiratory tract. Bronchial-associated lymphoid tissue (BALT) forms part of a common mucosal immune system (see Chapter 14). Unlike gut-associated lymphoid tissue, BALT does not form discrete structures in healthy subjects, but develops in response to repeated or persistent infection or other injurious stimuli such as smoking. Under these circumstances, BALT becomes organized in follicles and consists mainly of B cells, but distinct sites of collections of T cells are found on the periphery of these follicles. Epithelium overlying the BALT is devoid generally of cilia and goblet cells but displays membranous projections into the lumen, suited to selective antigen sampling, like the counterpart in the intestine – the M cell (see Chapter 14). Antigens are transported into the follicle where they can stimulate antigen-specific T- and B cells. Subsequently, IgA precursor B cells migrate into lymphatics and thence to the blood; bronchial IgA-bearing cells recirculate through both gut and lungs, so dispersing antigen-sensitive cells.
There are also large numbers of less organized lymphocytes in the lung within the pulmonary vasculature, in the lung interstitium and in the bronchoalveolar space.
Although relatively few lymphocytes are seen on routine sections, when calculated for the whole lung, lymphocyte numbers are similar to the circulating blood pool, i.e. about 10 × 109 lymphocytes.
Plasma cells producing IgE and IgG are also found in the bronchial tract. The physiological reason for the presence of IgE cells in the lung is unknown; it may even be an evolutionary accident, since the respiratory tract is a foregut derivative and IgE has a role in expulsion of intestinal parasites. While its physiological function is unclear, IgE is implicated in immediate (type I) hypersensitivity mechanisms (see Chapter 4) responsible for allergic asthma and hay fever.
We have considered respiratory diseases under four headings: infection, granulomatous disease, interstitial lung disease and vasculitis. Allergic diseases, which constitute the major immunological airways diseases, are considered in Chapter 4.
13.2 Respiratory infections
Infectious processes within the respiratory tract usually affect either the airways (e.g. rhinitis, sinusitis, laryngitis, bronchitis) or alveoli (pneumonia), although pneumonia can develop as a consequence of airways infection, particularly the small airways (bronchopneumonia).
Most respiratory tract infections reflect an interaction between virulent microorganisms and a relatively normal respiratory tract, whose protective mechanisms have been overcome either by the organism or by other injurious factors such as smoking or malnutrition. The respiratory tract is, however, also the most common site for infection to develop in immunodeficient subjects, and compromised immunity must be considered in all patients who present with serious, persistent, unusual or recurrent infections.
13.2.1 Infection in the immunocompetent host
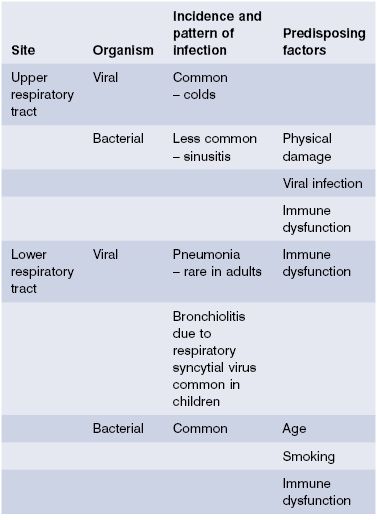
The airways are a major target for viral infection (Table 13.1), manifested most typically as the common cold, caused by many different viruses. These viruses usually replicate better in the cooler upper airways than at normal body temperature. Infection is probably cleared largely by virus-specific cytotoxic T cells.
Bacterial infection of the airways is less common and often occurs as a result of suppression of defence mechanisms by prior viral infection. The transient susceptibility of the airways to infection induced by some viral infections is only partially understood but includes physical factors such as inhibition of ciliary function and damage to the airway epithelium (e.g. influenza), and more subtle immunological mechanisms such as reduced expression of MHC molecules (adenoviruses) and inhibition of cytokine production [e.g. measles inhibits interleukin (IL)-12 production]. The most striking example is influenza A, which can lead to devastating pneumonia, usually caused by Staphylococcus aureus, in debilitated patients. However, the most important causes of pneumonia in the immunocompetent host are bacterial (Table 13.1).
Bacterial pneumonia is a common problem. It accounts for up to 10% of hospital admissions in developed countries and carries a considerable mortality: in the UK, pneumonia still causes 10 times as many deaths as all other infectious diseases together. While most of these deaths occur as a final event in debilitated patients, some previously healthy children and adults also die of pneumonia in spite of seemingly appropriate antibiotic therapy. The most devastating consequences of pneumonia are seen in developing countries. Each year, about 5 million children die of pneumonia before they are 5 years old. In South American countries, for instance, infant mortality from pneumonia and influenza is approximately 30 times greater than in the USA. Pneumococcal infections account for the majority of bacterial pneumonias and carry a mortality rate of 6–30%. Immunization against pneumococcal infections with polyvalent conjugate vaccines has significantly reduced the invasive disease in young children. However, pneumococcal disease remains a notable cause of mortality in the developing world and community-acquired pneumococcal pneumonia remains a problem in adults in the developed world despite pneumococcal polysaccharide immunisation. A recent Canadian study showed that the polysaccharide vaccine was not effective in patients with chronic obstructive airways disease who were 65 years of age or older, but it reduced the risk of acquiring pneumonia by 80% in younger patients. Hospital admission rates and lengths of stay were lower in the vaccine group.
 Case 13.1 Pneumonia and chronic lymphatic leukaemia [CLL]
Case 13.1 Pneumonia and chronic lymphatic leukaemia [CLL]A 65-year-old man was admitted with bilateral lower-lobe pneumonia. He had felt exhausted for 6 months, had lost 3 kg in weight and suffered 4 chest infections that responded to antibiotics. He did not smoke. He was clinically anaemic but had no finger clubbing, lymphadenopathy or splenomegaly. On investigation, he had a low haemoglobin (92 g/l) and a raised erythrocyte sedimentation rate (ESR) (84 mm/h). The white cell count was very high (98 × 109/l) and 95% of these were lymphocytes. The platelet count was normal. Serum immunoglobulins were all low: IgG 3.2 g/l (NR 7.2–18.0), IgA 0.6 g/l (NR 0.8–5.0) and IgM 0.3 g/l (NR 0.5–2.0); no paraprotein bands were seen.
A provisional diagnosis of pneumonia complicating chronic lymphatic leukaemia was made and CLL was confirmed by surface marker studies, which showed that 98% of peripheral lymphocytes were monoclonal B cells (Chapter 6).
Sputum cultures grew untypable Haemophilus influenzae. Treatment with amoxycillin resulted in rapid clearing of the pneumonia but, in view of his high lymphocyte count and mild anaemia, he was started on chlorambucil to control the lymphoproliferation. He lacked detectable serum antibodies and failed to make IgG antibodies to pneumococci on immunization; furthermore, all three major classes of serum immunoglobulins were low. Prophylactic IgG replacement therapy was started at a dose of 0.4 g/kg body weight per month, and he remained well for the next 5 years.
13.2.2 Infection in the immunocompromised host
Serious or recurrent infection does not always reflect disordered immunity (Table 13.2). The abnormal mucosal environment in cystic fibrosis is a potent predisposing cause for infection, as is bronchial obstruction due to factors ranging from tumours to plugging by mucus. Recurrent bronchial inflammation from many causes, particularly when associated with obstruction, leads to the development of bronchiectasis: dilated, damaged bronchi which themselves predispose to further infection, thus amplifying and perpetuating the process. However, there are certain infections or patterns of infection that should always lead to the consideration of underlying immunodeficiency (Fig 13.2). Some of these infections (such as Pneumocystis pneumonia) are virtually always associated with underlying immunodeficiency, whereas chronic sinopulmonary infection is associated with antibody deficiency in only 5% of cases. Nevertheless, the highly treatable nature of many immunodeficiency states (particularly antibody deficiencies) makes investigation mandatory (see Chapter 3).
Table 13.2 Important non-immunological causes of recurrent or severe infection
Airway obstruction
|
Mucociliary dysfunction
|
Fig. 13.2 Patterns of respiratory infection associated with specific immunodeficiencies. CMV, Cytomegalovirus; HIV, human immunodeficiency virus.
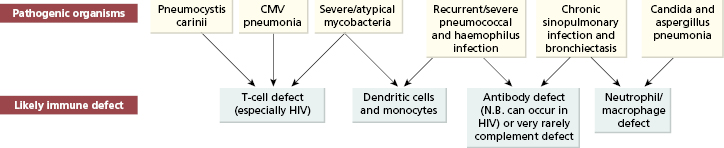
In adults, secondary causes of immunodeficiency are more common than primary ones and should be excluded first (see Chapter 3). An insidious pneumonic illness with dry cough, dyspnoea and fever may be caused by Pneumocystis carinii, cytomegalovirus (CMV) or atypical mycobacterial infection and should raise the possibility of acquired immune deficiency syndrome (AIDS) (see Chapter 3). Opportunistic viral, fungal and protozoal infections are common in patients with AIDS or iatrogenic defects in cell-mediated immunity, but they also suffer from common bacterial infections. Patients with AIDS frequently have infections with several microorganisms simultaneously.
13.3 Granulomatous diseases
The granulomatous diseases are a heterogeneous group of disorders with differing aetiologies, clinical presentations, histological characteristics and responses to therapy.
13.3.1 Formation of a granuloma
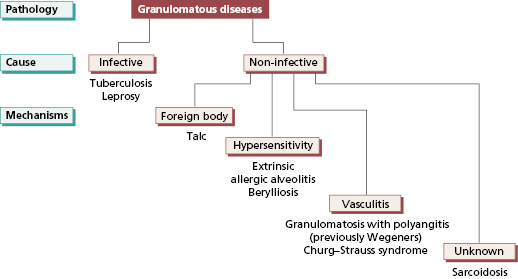
A granuloma is a histological structure made up largely of macrophages that have differentiated into epithelioid cells and often also fused to form multinucleate giant cells. Granulomas form in the presence of an antigen or foreign substance that cannot be easily broken down or eliminated; they can be regarded as a mechanism for containing and possibly destroying that antigen or foreign body, and may be reversible if antigen is destroyed only slowly. However granuloma formation is frequently associated with increased deposition of fibrous tissue. This fibrosis, which is irreversible, is often the most troublesome feature of granulomatous diseases. Granulomatous diseases can be both infective and non-infective (Fig 13.3).
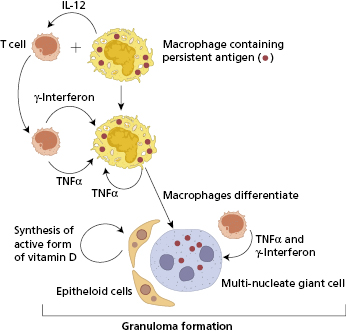
The most important immunological pathway leading to granuloma formation involves CD4+ T-cell-dependent activation of macrophages (type IV hypersensitivity). The presence of a suitable intracellular antigen, such as mycobacterial cell wall, induces the production of IL-12. This cytokine then stimulates the development of a Th1 T-cell response, with production of cytokines such as such as interferon (IFN)-γ. The process appears to be sustained and perpetuated by IFN-γ, IL-12 and other cytokines, in particular tumour necrosis factor (TNF)-α, produced by both T cells and macrophages themselves (Fig 13.4). The key role of TNF-α in sustaining this process is shown by the reduction or resolution of granuloma by anti-TNF agents. Although useful therapeutically in the treatment of Crohn’s disease and sarcoidosis, these agents also cause an increased risk of tuberculosis. Epithelioid cells also produce fibrogenic cytokines such as transforming growth factor (TGF)-β and can synthesize the active form of vitamin D from inactive precursors. Active vitamin D plays an important role in stimulating macrophage differentiation within the granuloma. In some granulomatous disorders, sufficient active vitamin D is produced to cause hypercalcaemia.
Granulomas also form in some diseases dominated by a Th2 pattern of cytokine production, such as schistosomiasis and the Churg–Strauss syndrome, though the mechanism in these disorders is less clear.
 Case 13.2 Pulmonary tuberculosis
Case 13.2 Pulmonary tuberculosisA 23-year-old man presented with a 4-week history of coughing, breathlessness and malaise. He had lost 4 kg in weight, but had no history of night sweats or haemoptysis. He had returned from holiday in Pakistan 2 months earlier. On examination, he was mildly pyrexial (37.8 °C) but had no evidence of anaemia or clubbing. Crepitations were audible over the lung apices; there were no other physical signs. His haemoglobin and white cell count were normal but the C-reactive protein (CRP) was 231 mg/l. The chest X-ray showed bilateral upper- and middle-lobe shadowing but no hilar enlargement. Sputum was found to contain acid-fast bacilli and Mycobacterium tuberculosis was confirmed by PCR and subsequently cultured. A diagnosis of pulmonary tuberculosis was made. The patient was treated with isoniazid and rifampicin for 6 months, together with pyrazinamide for the first 2 months. He was allowed home on chemotherapy when his sputum became negative on direct smear. The chest X-ray is now much improved.
13.3.2 Tuberculosis
The global impact of infection with Mycobacterium tuberculosis is enormous. Around one-third of the world’s population is infected with the tubercle bacillus and about 1.5 million people die every year from the consequences of this infection. The greatest part of this burden of disease falls upon the developing world. Tuberculosis is a disease driven by poverty and poor nutrition. Immunosuppression associated with HIV infection has combined with these older risk factors to increase the incidence of tuberculosis (Table 13.3). The impact of tuberculosis in Western Europe and North America is minimal by comparison, and has fallen considerably over the last 50 years, though this downward trend has stopped now. This is due to a combination of HIV infection, increased mobility of populations across the world and worsening pockets of poverty even in the richest of nations. Other immunosuppressive factors should not be forgotten, particularly poor nutrition and the immunosuppressive effect of infections such as measles. Vitamin D deficiency (due to diet or lack of exposure to sunlight) may be an important cofactor, reflecting the role of this vitamin within granulomas. The outcome of exposure to M. tuberculosis depends on several factors: susceptibility genes play a significant role in as does the intensity of exposure to M. tuberculosis; high levels of exposure may overwhelm even vigorous immune responses.
Table 13.3 Risk factors for developing tuberculosis
1 Poverty and malnutrition 2 Close contact with patients with active infection 3 Immunosuppression (especially secondary to HIV infection or anti-tumour necrosis factor [-TNF] drugs) 4 Prolonged residence in countries with high prevalence of tuberculosis 5 Genetic predisposition |
Mycobacterium tuberculosis is a slow-growing bacterium with an inert, waxy cell wall. It produces no toxins and causes disease only by its stimulation of the body’s immune defence mechanisms coupled with an inexorable increase in numbers. The organism is rapidly taken up by alveolar macrophages. In most individuals, activation of dendritic cells results in a brisk Th1-pattern immune response leading to local granuloma formation in an area of lung and the draining lymph node. This contains and effectively eliminates the organism, although the immune system alone may never be able to clear M. tuberculosis completely. Once infection has occurred, the organism remains in a latent form if the host is untreated, even when disease does not develop. In a proportion of infected individuals, the T-cell response is less effective and the granulomatous response only partially contains the organism. An area of localized but progressive granulomatous inflammation develops which slowly enlarges and often cavitates due to a necrotic process called caseation, which is typical of tuberculous granulomas and which distorts surrounding structures by fibrosis. The Th1 response to M. tuberculosis may wax and wane over time: latent disease can reactivate if immunity is suppressed (by malnutrition, drugs or intercurrent infection) and become clinically evident treatment with anti-TNF agents specifically reduce resistance to reactivation, indicating the important role played by TNF in suppressing the infection.
If the T-cell response is defective, or skewed towards a Th2 pattern, then the organism reproduces freely in macrophages, and disseminated disease may develop. This is miliary tuberculosis (Fig 13.5), where many organs are involved by small collections of macrophages packed with mycobacteria with only partial evidence of granuloma formation. This is associated with marked systemic ill-health, due to cytokine release from macrophages. Cytokine release is driven partly by the ineffective T-cell response and partly by the direct effect of the mycobacterial cell wall on dendritic cells.
Fig. 13.5 Cut section of lung from a patient who died from miliary tuberculosis. Note the 2–5-mm nodules scattered through the lung tissue. ‘Miliary’ means ‘resembling millet seeds’.

Improvement in diet and living conditions, and more recently control of HIV infection, are the major public health measures that reduce the impact of tuberculosis. The introduction of prophylactic immunization with an attenuated form of mycobacterium, bacillus Calmette–Guérin (BCG), had a significant role in reducing invasive disease infection due to mycobacterial species in young children. It is used to protect hospital personnel and tuberculosis contacts, though evidence for efficacy is controversial. New vaccines are needed urgently.
The presence of a T-cell response to M. tuberculosis can be established clinically by injecting mycobacterial antigen intradermally and assessing the delayed skin reaction at 48–72 h (the basis of the Mantoux test: Fig 13.6). This is useful in assessing prior immunity before immunization, and may also be a helpful diagnostic pointer in active disease. However, such test are subjective and largely superseded by the interferon gamma release assay (IGRA) (Chapter 19). Diagnosis of active infection, however, is done by PCR of induced sputum or biopsy material, still confirmed by microscopy and culture (Case 13.2).
Fig. 13.6 Strongly positive Mantoux reaction 96 h after the intradermal injection of antigen from Mycobacterium tuberculosis.
Treatment regimens Treatment regimens comprise a combination of several antimicrobial agents (e.g. rifampicin, isoniazid, pyrazinamide and ethambutol) often administered for a period of 6 months. Unfortunately, recent years have seen the emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis strains. These resistant organisms necessitate treatment with second-line agents and are associated with high mortality rates. MDR and XDR tuberculosis loom as particularly daunting public health problems, hence the urgency for new vaccines. The BCG vaccine only partially protects against MDR and XDR; it is protective to non-tuberculous mycobacteriosis (NTM, MAC) because of BCG cross-reactivity.
13.3.3 Sarcoidosis
Sarcoidosis is a multisystem, granulomatous disorder most commonly affecting young adults of either sex. It is uncommon after the age of 40 years.
 Case 13.3 Sarcoidosis
Case 13.3 SarcoidosisA 36-year-old man complained of breathlessness on exercise for 6 months. He also had mild chest tightness and stiff joints but no skin or eye problems. There was no family history of chest disease and he had never been abroad. He had been immunized with BCG as a schoolboy. On examination, he had no clubbing and no abnormal chest signs. Investigations showed a normal haemoglobin (143 g/l), white cell count (4.4 × 109
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree