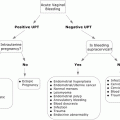
Symptom/sign
Incidence rate
Overall severity
Mild
58.2 %
Moderate
32.8 %
Severe
9.0 %
Flushing
54.7 %
Dyspnea
29.7 %
Chest discomfort
26.2 %
Pruritus
24.6 %
Elevated blood pressure
19.5 %
Back pain
16.8 %
Chills
15.6 %
Hypoxia
14.8 %
Nausea
14.1 %
Tachycardia
11.7 %
Hypotension
10.9 %
Taxanes
Paclitaxe l has the highest rate of hypersensitivity reactions among all taxanes, as up to 30 % of patients may experience major reactions without premedication (Levett et al. 2002). Major risk factors for hypersensitivity seem to be a fast infusion rate and short infusion schedule. Researchers have debated that a reason for the increased incidence of these reactions is that paclitaxel is insoluble and requires use of the solvent polyethoxylated castor oil (Kolliphor EL [formerly Cremophor EL]) for administration, which increases its toxicity and incidence of hypersensitivity episodes and entraps paclitaxel in micelles, decreasing its availability to tumor cells (Zhang et al. 2005; Weiss et al. 1990). In an attempt to reduce these effects, investigators have examined new paclitaxel formulations. One of these novel formulations that received U.S. Food and Drug Administration approval is nanoparticle albumin-bound paclitaxel (Abraxane). In a study of 175 patients, authors reported no Abraxane dose interruptions owing to hypersensitivity reactions (Socinski et al. 2010).
Most hypersensitivity reactions to taxanes occur within 2–10 min after the infusion starts and tend to improve within 15–30 min, and same-day rechallenge after improvement of symptoms usually does not lead to recurrence of the reactions. Minor reactions are much more common than major ones and include flushing, chest tightness, back pain, pruritus, and erythematous rash. Major hypersensitivity reactions include dyspnea, bronchospasm, hypotension, urticaria, and angioedema. We recommend premedication with steroids and antihistamines (H1 antagonists and H2 blockers) to reduce the incidence of major hypersensitivity reactions (1–3 %) (Levett et al. 2002).
Platinum-Based Agents
Although hypersensitivity reactions to carboplatin are less common than those to cisplatin, widespread use of the former agent for many different cancers makes these reactions a significant concern in ambulatory chemotherapy units. All platinum-based compounds cause type I reactions that seem to be mediated by IgE, and cross-reactivity among platinum analogs is possible. Cisplatin can cause hypersensitivity reactions in up to 20 % of patients. Furthermore, it may cause type II reactions. Anaphylaxis may occur in up to 5 % of patients without premedication. Oxaliplatin has caused hypersensitivity reactions in up to 12 % of infusions without premedication (Meyer et al. 2002). The most common symptoms of hypersensitivity reactions to these agents are rash (including urticaria), bronchospasm, and hypotension, but unlike with taxanes, reactions to platinum-based compounds can be very severe and even fatal if not treated aggressively.
Asparaginase
Hypersensitivity reactions to l-asparaginase usually occur after administration of the second dose, and the reaction risk increases with each subsequent dose, with rates of up to 33 % by the fourth cycle (Levett et al. 2002). Fortunately, anaphylaxis is not common (less than 10 % of cases), and fatal reactions are even rarer (less than 1 % of cases). New formulations such as pegylated asparaginase are covalently attached to polyethylene glycol and do not appear to cause anaphylactic reactions. In addition to the risk factors described above, use of high doses (more than 6000 IU/m2/day) and intravenous administration increase the risk of hypersensitivity reactions to asparaginase. Common symptoms of hypersensitivity to asparaginase include pruritus, dyspnea, agitation, rash/urticaria, angioedema, hypotension, laryngospasm/bronchospasm, nasal congestion, and pain. These are typically type I reactions and usually occur within 1 h after administration.
Alkylating Agents
The most commonly used classical alkylating agents are cyclophosphamide and ifosfamide, which cause type I reactions. However, the etiology of these reactions is unclear, as mesna, given together with ifosfamide, by itself can also cause type I reactions. Common symptoms include rash/urticaria, angioedema, and anaphylaxis. Chlorambucil and melphalan also cause type I hypersensitivity reactions, but they are rare unless melphalan is given intravenously (2–4 %). Chlorambucil can cause hemolysis, toxic epidermal necrolysis, and pneumonitis, and melphalan usually causes urticaria and angioedema (Levett et al. 2002). Procarbazine and dacarbazine are considered by most researchers to be nonclassical alkylating agents. Procarbazine can cause both type I and type III hypersensitivity reactions in up to 18 % of patients. The most common symptoms of type I reactions are urticaria and maculopapular rash, whereas that of type III reactions is allergic alveolitis .
Anthracyclines
Authors have not reported any hypersensitivity reactions to idarubicin, but daunorubicin and doxorubicin can cause type I reactions. Pruritus, erythematous rash, urticaria, hypotension, and anaphylaxis are common symptoms, but cross-reactivity seems to be uncommon.
Antimetabolites
IgE-mediated hypersensitivity reactions to cytarabine are rare, with symptoms including chest pain, dyspnea, angioedema, urticaria, fever, and hypotension. Another potential reaction to administration of this drug is cytarabine syndrome (neutrophilic eccrine hidradenitis and palmar-plantar erythema). Intrathecal administration of liposomal cytarabine has not been associated with any hypersensitivity reactions (Benesch et al. 2009). Because 6-mercaptopurine and its imidazole analog azathioprine are used more frequently for conditions such as arthritis and inflammatory bowel disease than for cancer, most data on hypersensitivity reactions to it are derived from studies of the former conditions. Furthermore, concurrent use of steroids is common in the treatment of these conditions and may have influenced the reaction rates reported in the literature. The rate of hypersensitivity reactions (excluding pancreatitis) for 6-mercaptopurine was 2.7 % in a large series, with the most common symptoms being fever, arthralgia, and back pain (Korelitz et al. 1999). Most patients will have recurrence of these hypersensitivity reactions during rechallenge, and cross-reactivity with azathioprine is expected. Common symptoms of hypersensitivity reactions to azathioprine include fever, urticaria, rash, arthralgia, and dyspnea. Fortunately, hypotension and distributive shock occur very infrequently. The mechanism of these reactions is unknown, but investigators believe that these agents or their metabolites may function as haptens and bind to protein molecules to induce type I hypersensitivity reactions.
Topoisomerase Inhibitors
Hypersensitivity reactions to topoisomerase inhibitors are more common in children than in adults, more common for teniposide than for etoposide (7 % versus 3 %), and more common when given intravenously (etoposide) than via other routes. These can be type I (urticaria, angioedema, bronchospasm, hypotension, flushing, and rash) or type II (particularly hemolytic anemia with teniposide) reactions. However, etoposide is insoluble, requiring the use of solvents such as benzyl alcohol, Kolliphor EL, and polysorbate 80 (Tween 80), which by themselves may play a role in causing hypersensitivity reactions. The symptoms of these reactions can occur during administration of the first dose and within the first 10 min after infusion. Patients should not undergo rechallenge if these symptoms are severe or slow to resolve.
Miscellaneous Chemotherapy
Methotrexate can cause both type I (rash, urticaria, pruritus, angioedema, and hypotension) and type III (pneumonitis, pleural effusions, lung eosinophilia, hilar adenopathy, and cutaneous vasculitis) hypersensitivity reactions. Mitomycin C can cause delayed type IV reactions when given intravesically in up to 10 % of patients. Symptoms include erythematous, vesicular, and pruritic rash and angioedema. Skin reactions can be useful in predicting hypersensitivity reactions. Fluorouracil rarely causes type I reactions, consisting of angioedema and hypotension. In addition, bleomycin rarely causes fatal reactions but usually causes urticaria, bronchospasm, and periorbital edema. Use of a test dose of 1 mg of bleomycin is recommended. Mitoxantrone can cause erythematous rash and angioedema, and rechallenge can cause reaction recurrence (Levett et al. 2002).
Management Algorithm
The keys to optimal management of hypersensitivity reactions are prophylaxis, clinical staff training and patient education on potential symptoms of the reactions to shorten time to treatment, and desensitization, if recommended. For high-risk chemotherapeutic agents, prophylaxis can be administered with premedication in the form of 20 mg of dexamethasone, 50 mg of diphenhydramine, or 300 mg of cimetidine (or other H2 blockers). The typical desensitization regimen for paclitaxel consists of 20 mg of dexamethasone given orally every 6 h for 4 doses followed by the prophylactic regimen described above.
Figure 11.1 shows the algorithm used in our institution for treatment of hypersensitivity reactions . It comprises management recommendations that should be adapted to each patient’s diagnosis and comorbid conditions. The first step in the algorithm is to monitor the patient for any signs or symptoms of hypersensitivity and stop the infusion if any are detected. If the patient is unresponsive, a “code” should be called, and his or her vital signs should be monitored every 5 min. If fever, chills, and/or rigor develop, 1000 mg of acetaminophen should be given unless the patient is a stem cell transplant recipient or has received acetaminophen within the last 4 h. If chills persist, administration of a low dose of meperidine should be considered. Patients also should be monitored for signs of hypoxia, with oxygen supplementation given as needed. If the patient experiences flushing, pruritus, rash, or hives, 50 mg of diphenhydramine should be given intravenously over 2 min. For more severe symptoms such as hypotension, shortness of breath with wheezing or stridor, and swelling of the face, lip, or tongue, the following should be given: (1) normal saline at 150 mL/h, (2) 0.5 mL of epinephrine (1:1000) subcutaneously, (3) 50 mg of diphenhydramine intravenously over 2 min, and (4) 100 mg of hydrocortisone intravenously over 1 min. Patients who have reactions to paclitaxel (diluted in Kolliphor EL) usually have good prognoses despite frequently presenting with severe symptoms. These patients typically present with flushing, chest and/or back tightness, hypoxia, shortness of breath without stridor or wheezing, and hypertension or hypotension, and they should be given 50 mg of diphenhydramine and 100 mg of hydrocortisone, only adding epinephrine if their symptoms worsen or do not improve within 15–30 min after discontinuation of the infusion. In our institution, only 3.2 % of patients with hypersensitivity reactions have been transferred to our emergency center, 2.0 % have been admitted to the hospital, 84.0 % have undergone rechallenge the same day (88.1 % with no further reactions), and 94.8 % have been discharged.
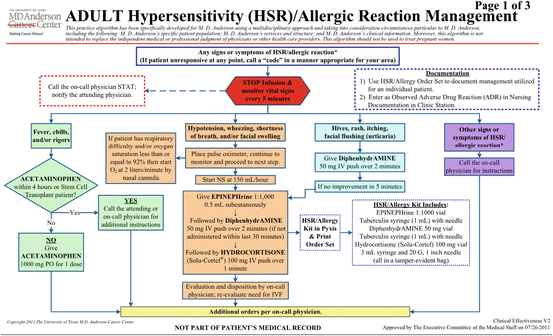

Fig. 11.1
Algorithm used at MD Anderson for treatment of hypersensitivity reactions. NS normal saline, IV intravenous, IVF intravenous fluid
Systemic Reactions to Monoclonal Antibodies
Administration of monoclonal antibodies frequently may lead to massive release of cytokines. The most common symptoms of systemic reactions to these antibodies are fever and rigor. Occasionally, they may lead to dyspnea, hypoxia, hypotension, and, rarely, death. The treatment of these reactions is similar to that of hypersensitivity reactions to chemotherapeutic agents. Systemic symptoms specific to individual agents are described below.
Denileukin Diftitox
Denileukin diftitox works by binding to the interleukin-2 receptor and delivering diphtheria toxin to lymphoma cells, inhibiting cellular protein synthesis and leading to cell death. Severe reactions to denileukin diftitox occur in 2 % of patients and consist of rash, dysphagia, back pain, shortness of breath, tachycardia, chest pain, vasodilatation, and syncope (Levett et al. 2002). The treatment of these reactions is similar to that of hypersensitivity reactions and includes discontinuation of the drug and intravenous administration of antihistamines, hydrocortisone, and epinephrine as necessary.
Another significant reaction to the use of denileukin diftitox is capillary (vascular) leak syndrome. Its symptoms, which usually occur within 2 weeks after infusion, consist of vascular leakage, hypoalbuminemia, severe edema, pleural effusion, and hypotension. Only supportive care for it is necessary, which may include intravenous fluids, diuretics, and albumin infusion. Further infusions of denileukin diftitox should be delayed until the patient’s albumin level is greater than 3.0 g/dL, if possible. Premedication with corticosteroids is recommended.
Trastuzumab
The HER-2 proto-oncogene is overexpressed in up to 35 % of breast cancer cases. Trastuzumab is a humanized monoclonal antibody against HER-2/neu (c-erb B2) protein that is frequently used in the treatment of metastatic breast cancer. Fever and chills are the most common symptoms of reactions to trastuzumab during the first infusion; other effects include nausea, vomiting, diarrhea, severe myalgia, and pain at the tumor site (Ewer et al. 2005). Supportive treatment, including acetaminophen, opiates (e.g., meperidine), antiemetics, and antidiarrheals, should be considered.
Rituximab
Used commonly in the treatment of lymphomas, leukemias, and some rheumatologic conditions, rituximab is a humanized murine monoclonal antibody directed against CD-20, a B-cell-specific surface molecule. Reactions to rituximab are very common and have occurred in up to 84 % of patients in some trials (Oldham 1983). Most symptoms of these reactions usually occur 30–120 min after the first infusion and are mild, including transient fever, chills, headache, nausea, and fatigue. Premedication regimens often include diphenhydramine and acetaminophen to ameliorate the symptoms. If reactions occur, the infusion of rituximab should be interrupted and then restarted at half the initial infusion rate once the symptoms resolve. Supportive treatment is recommended, and most symptoms resolve with interruption or slowing of the infusion. Serious effects of reactions to treatment with rituximab may be related to immune response to the use of a humanized murine antibody and include pain at the tumor site, bronchospasm, arrhythmias, hypotension, rash, and even angioedema. The treatment of these symptoms is similar to that of hypersensitivity reactions and includes discontinuation of the drug and intravenous administration of antihistamines, hydrocortisone, and epinephrine as necessary.
Gemtuzumab Ozogamicin
Used in the treatment of acute myeloid leukemia, usually when the patient may not be able to tolerate more cytotoxic chemotherapy, gemtuzumab ozogamicin is a humanized anti-CD33 antibody-calicheamicin conjugate that causes DNA double-strand breaks and apoptosis. Like with other monoclonal antibodies, the most common symptoms of reactions to gemtuzumab ozogamicin are fever and chills. Other symptoms include nausea, shortness of breath, and hypotension. These symptoms usually occur within 4 h after infusion. Pretreatment prophylactic regimens for reactions to this agent include acetaminophen and diphenhydramine given 15–30 min prior to infusion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree